For extraction of benzoic acid from water into toluene with toluene the dispersed phase, we measure the
Question:
For extraction of benzoic acid from water into toluene with toluene the dispersed phase, we measure the following concentrations of benzoic acid: \(\mathrm{C}_{\mathrm{D}, \text { in }}=0, \mathrm{C}_{\mathrm{D}, \text { out }}=0.00023\), and \(\mathrm{C}_{\mathrm{C}, \text { out }}=0.00536\) with concentrations in \(\mathrm{mol} / \mathrm{m}^{3}\). The mixer is \(0.75-\mathrm{m}\) tall and has a \(0.75-\mathrm{m}\) diameter. Flow rate of the dispersed phase is \(Q_{D}=0.0012 \mathrm{~m}^{3} / \mathrm{s}\) and \(Q_{C}\) \(=0.097 \mathrm{~m}^{3} / \mathrm{s} . \varphi_{\mathrm{d}}=0.09\). Data for density, equilibrium, and molecular weight are in Example 16-5.
Example 16-5
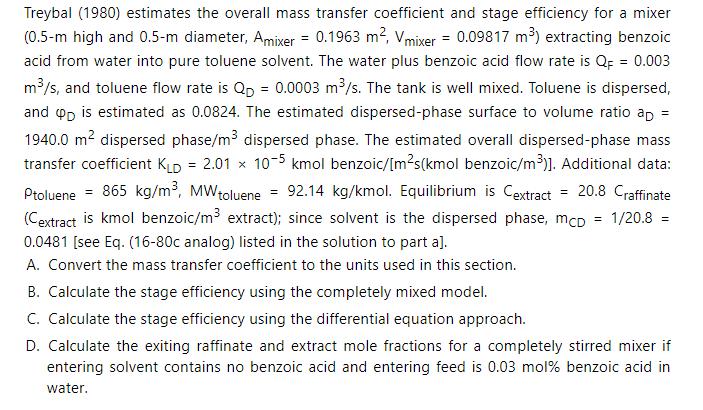 a. Determine stage efficiency \(\mathrm{E}_{\mathrm{MD}, \text { Conc }}\).
a. Determine stage efficiency \(\mathrm{E}_{\mathrm{MD}, \text { Conc }}\).
b. Calculate value of \(\mathrm{K}_{\mathrm{O}-\mathrm{ED}} \mathrm{a}\) in concentration units using the completely mixed staged model.
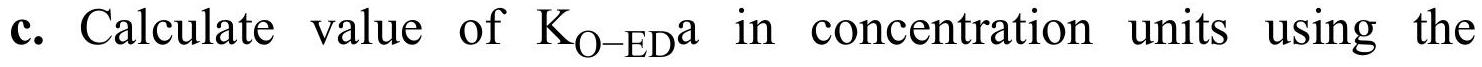
differential equation model.
d. If by accident the value of \(\mathrm{K}_{\mathrm{O}-\mathrm{ED}} \mathrm{a}\) calculated using the completely mixed staged model is used to predict stage efficiency using the differential equation model, what (incorrect) value of \(\mathrm{E}_{\mathrm{MD}, \mathrm{Conc}}\) is obtained?
This problem is a clone of Problem 16.D18 but with a different value for continuous-phase concentration and in different units.
Problem 16.D18
For extraction of benzoic acid from water into toluene with toluene the dispersed phase, we measure the following mole fractions of benzoic acid: \(\mathrm{x}_{\mathrm{D}, \text { in }}=0, \mathrm{x}_{\mathrm{D}, \text { out }}=0.00023\), and \(\mathrm{x}_{\mathrm{C}, \text { out }}=1.99 \times 10^{-6}\). The mixer is \(0.75 \mathrm{~m}\) tall and has a \(0.75 \mathrm{~m}\) diameter. Flow rate of the dispersed phase is \(\mathrm{Q}_{\mathrm{D}}=0.0012 \mathrm{~m}^{3} / \mathrm{s}\) and \(\mathrm{Q}_{\mathrm{C}}=0.097 \mathrm{~m}^{3} / \mathrm{s}\). Data for density, equilibrium, and molecular weight are in Example 16-5.

a. Determine stage efficiency \(\mathrm{E}_{\mathrm{MD}, \text { mole_frac }}\). Note: The unit conversion in Eq. (16-94) is required to calculate \(\mathrm{m}\) for equilibrium, \(\mathrm{y}=\mathrm{mx}\), in mole fractions.
Eq.16-94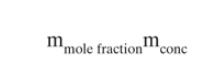 b. Calculate value of \(\mathrm{K}_{\mathrm{O}-\mathrm{ED}} \mathrm{a}\) using the completely mixed staged model.
b. Calculate value of \(\mathrm{K}_{\mathrm{O}-\mathrm{ED}} \mathrm{a}\) using the completely mixed staged model.
c. Calculate value of \(\mathrm{K}_{\mathrm{O}-\mathrm{ED}} \mathrm{a}\) using the differential equation model.
d. If by accident the value of KO−EDa calculated using the completely mixed staged model is used to predict the stage efficiency using the differential equation model, what (incorrect) value of EMD,mole_frac is obtained?
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





