For the formation of glucose from cellulose discussed in Problem 6.12, the rate constant k 1 shows
Question:
For the formation of glucose from cellulose discussed in Problem 6.12, the rate constant k1 shows the following temperature dependence at an HCl concentration of 0.055 mol/L: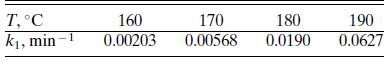
Check the validity of the Arrhenius relation and determine the activation energy.
Problem 6.12
The formation of sugars from biomass is of considerable interest in a variety of applications, including energy alternatives to fossil fuel. Harris and Kline studied the formation of glucose from cellulose obtained from Douglas Fir in 1949, carrying out the reaction at a number of temperatures in the presence of HCl.
a. The rate was found to be first order in cellulose (A) concentration (r = k1cA), but dependent on HCl concentration as shown in Table 6.P6. Find the dependence of the rate on HCl.
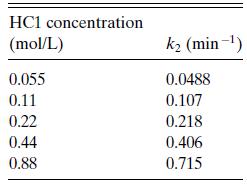
b. Glucose also decomposes in the presence of HCl. Harris and Kline reported the decomposition reaction to be first order in glucose (rglucose− =k2cglucose), with a first-order rate constant at 190◦C that depended on HCl as shown in Table 6.P7. Can you find a rate expression in a simple form?
Table 6.P6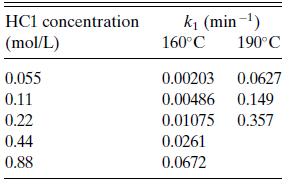
Table 6.P7
Step by Step Answer:






