Question: For the voltammogram in Problem 5.3, determine whether the one-electron transfer process is reversible in the electrochemical sense. Data from Problem 5.3 From the CV
For the voltammogram in Problem 5.3, determine whether the one-electron transfer process is reversible in the electrochemical sense.
Data from Problem 5.3
From the CV below, estimate the formal potential for the redox couple.
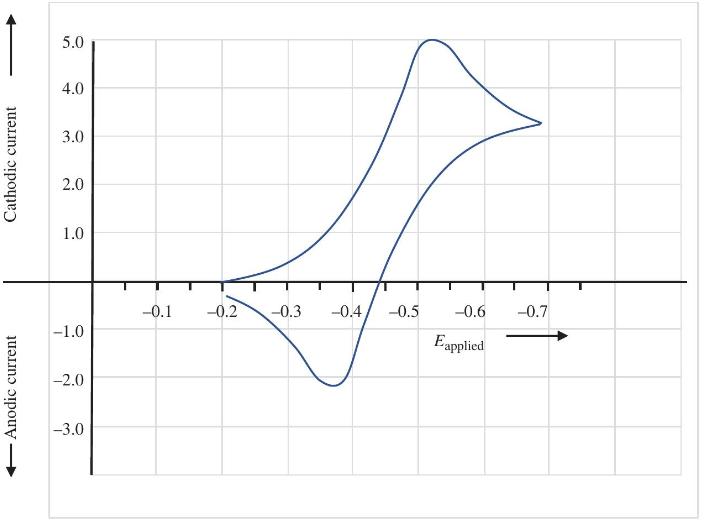
Anodic current Cathodic current 5.0 4.0 3.0 2.0 1.0 -0.1 -0.2 -0.3 -0.4 -0.5 -0.6 -0.7 -1.0 Eapplied -2.0 -3.0
Step by Step Solution
3.50 Rating (163 Votes )
There are 3 Steps involved in it
For a reversible s... View full answer

Get step-by-step solutions from verified subject matter experts


