Question: Does the voltammogram in Problem 5.3, indicate any possibility that the reduced or the oxidized form is unstable under the conditions in this solution? Support
Does the voltammogram in Problem 5.3, indicate any possibility that the reduced or the oxidized form is unstable under the conditions in this solution? Support your answer with a calculation.
Data from Problem 5.3
From the CV below, estimate the formal potential for the redox couple.
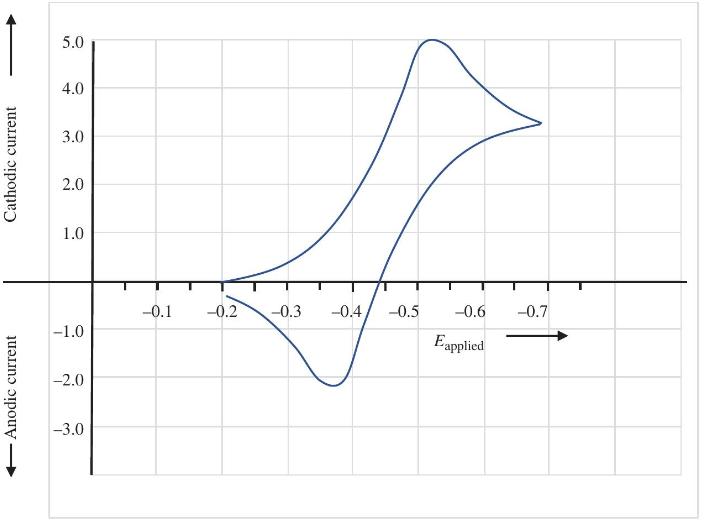
Anodic current Cathodic current 5.0 4.0 3.0 2.0 1.0 -0.1 -0.2 -0.3 -0.4 -0.5 -0.6 -0.7 -1.0 Eapplied -2.0 -3.0
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it
Figure 513C If the two redox forms are stable on the time scale of the experiment then ... View full answer

Get step-by-step solutions from verified subject matter experts


