In an experiment carried out by one of our undergraduate students, a water stream and a chloroform
Question:
In an experiment carried out by one of our undergraduate students, a water stream and a chloroform stream** were fed continuously to a 1,000 cm3 cylindrical tank with a cross-sectional area of 81 cm2. The exit was located at one-fourth of the total height. Ammonia in amounts up to 1.7 g/L was dissolved in the chloroform feedstream and extracted by the water stream. The chloroform stream is denoted as Phase I and the water stream as Phase II. The equilibrium distribution coefficient for ammonia is M = cAeI/cAeII = 0.044 at room temperature over the range studied. It can be assumed that the densities of the streams are independent of the ammonia concentration, with ρI = 1,470 kg/m3, ρII = 1,000 kg/m3. The fractional approach to equilibrium, Ef, was measured in the water effluent as a function of the mixer RPM, as shown in Table 10.P4.
a. At 0 RPM the phase interface in the tank is nearly a horizontal plane. Estimate the overall mass coefficient, Km. Compare with the value obtained in the batch salt experiment in Section 10.3.3. What assumptions have you made?
b. Correlate Kma with RPM for this system, and in that way obtain a correlation for the efficiency of the mixer. Can you use your estimate of Km from part (a) to obtain a meaningful correlation for the surface area?
c. Suppose that you wish to add another species to the aqueous phase that reacts with ammonia and is insoluble in chloroform. Can any of the terms in Equation 10.39 be neglected? Does this situation correspond to either of the limiting cases?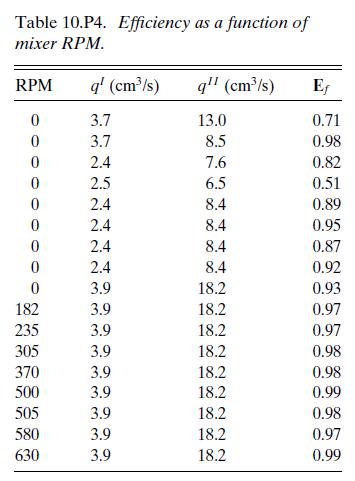
Step by Step Answer:






