Kinetic data for the reaction between sulfuric acid and diethyl sulfate are given in Examples 6.1 and
Question:
Kinetic data for the reaction between sulfuric acid and diethyl sulfate are given in Examples 6.1 and 6.4. Suppose that V = 25.4 L, qAf = qBf, cAf = 11.0 g-mol/L, cBf = 5.5 g-mol/L, and the required effluent concentration of sulfuric acid is cA = 4.0 g-mol/L. Find the flow rates
(a) Assuming that that the reaction may be taken to be irreversible
(b) Taking the reverse reaction into account.
Examples 6.1
The data in Table 6.1 were obtained for the reaction of sulfuric acid with diethyl sulfate, H2SO4 + (C2H5)2SO4 → 2C2H5SO4H, in aqueous solution at 22.9◦C. The initial concentrations of both reactants were the same, 5.5 g-mol/L. What is the rate expression?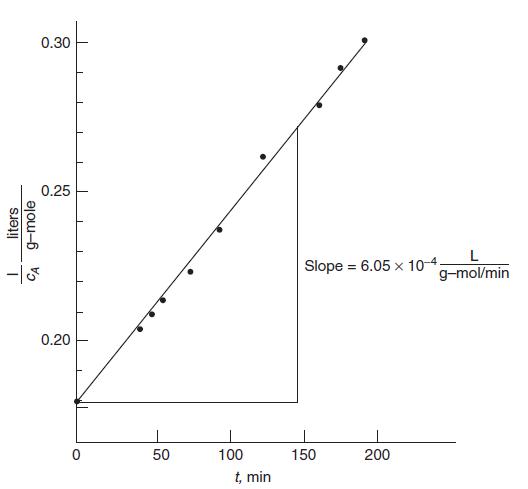
Figure 6.3. Computation of the second-order rate constant for the reaction between sulfuric acid and diethyl sulfate in aqueous solution.
In our symbolic nomenclature, H2SO4 is denoted by A, (C2H5)2SO4 is denoted by B, and 2C2H5SO4H is denoted by M. cA0 = cB0 = 5.5 g-mol/L.
μ = 2 and ψ = 0. We assume mass action kinetics, so the rate with equal initial concentrations will be of the form r = kc2A .* As required by Eq. 6.11a, the data are plotted in Figure 6.3 as 1/cA versus t. There is some experimental scatter, but the data are clearly represented by the straight line shown in the figure. The rate constant is then determined from the slope to be k = 6.05×10−4 L/(g-mol min).
Table 6.1
Concentration of H2SO4 versus time for the reaction of sulfuric acid with diethyl sulfate in aqueous solution at 22.9◦C. Data of Hellin and Jungers, Bull. Soc. Chim. France, No. 2, pp. 386–400 (1957).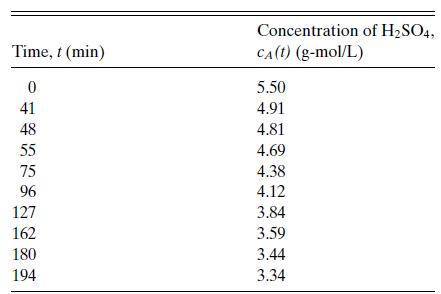
Equation 6.11a
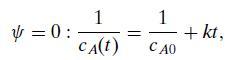
Examples 6.4
The cleavage of diacetone alcohol in the presence of hydroxide ion (e.g., aqueous NaOH) at 25◦C appears to be a simple first-order decomposition: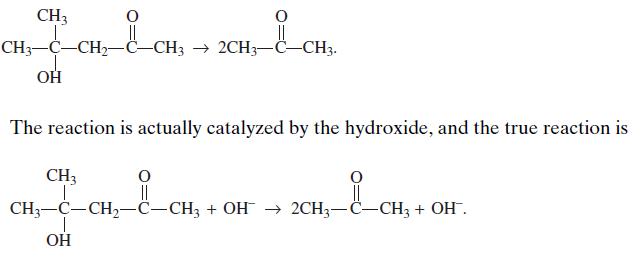
Data for the apparent first-order reaction-rate constant are shown in the first two columns of Table 6.4 for various concentrations of NaOH in the reaction mixture. Show that the reaction exhibits mass action kinetics with a true rate r = kcAcB. The third column in the table shows the pseudo-first-order rate constant k′ divided by the concentration of NaOH. The result is a constant value to within 3 percent, indicating that the true reaction rate is r = kcAcB, where k = 0.47 L/(g-mol min).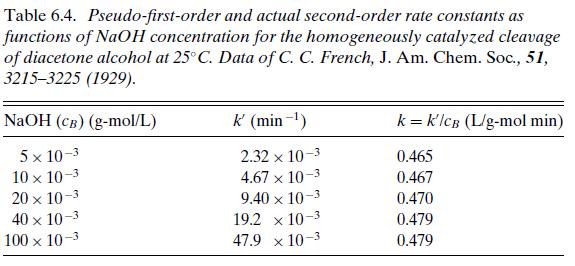
Step by Step Answer:






