Question: Consider the full data set in Table 6.5 for the reversible reaction between sulfuric acid and diethyl sulfate and test the applicability of each of
Consider the full data set in Table 6.5 for the reversible reaction between sulfuric acid and diethyl sulfate and test the applicability of each of the following rate expressions: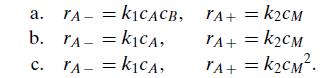
Table 6.5
Concentration of H2SO4 versus time for the reaction of sulfuric acid with diethyl sulfate in aqueous solution at 22.9◦C, full data
set. Data of Hellin and Jungers, Bull. Soc. Chim. France, No. 2, pp. 386–400 (1957).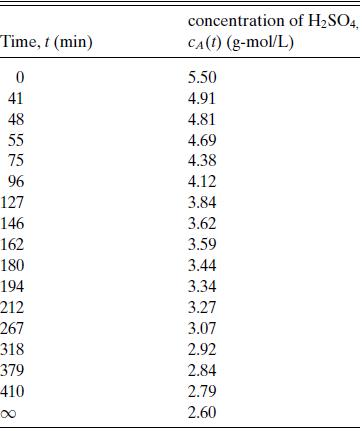
TAKICACB, = a. b. TAKICA, = C. TA- = k1CA, - = KCM TA+ = TA+ = KCM TA+ = KCM.
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
In order to test the applicability of each rate expression for the reversible reaction between sulfuric acid H2SO4 and diethyl sulfate one must analyze how the rate of reaction changes with the concen... View full answer

Get step-by-step solutions from verified subject matter experts


