Question: The data in Table 6.P3 are for reactant A in the irreversible reaction A + B products. The initial concentrations are believed to be
The data in Table 6.P3 are for reactant A in the irreversible reaction A + βB → products. The initial concentrations are believed to be in stoichiometric balance. Estimate the reaction order and find the rate constant.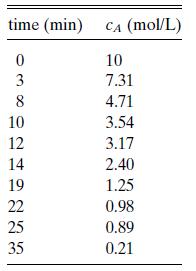
time (min) CA (mol/L) 0 10 3 7.31 8 4.71 3.54 3.17 2.40 1.25 10 12 14 19 22 25 35 0.98 0.89 0.21
Step by Step Solution
3.31 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


