Two identical large glass bulbs are filled with gases and connected by a capillary tube that is
Question:
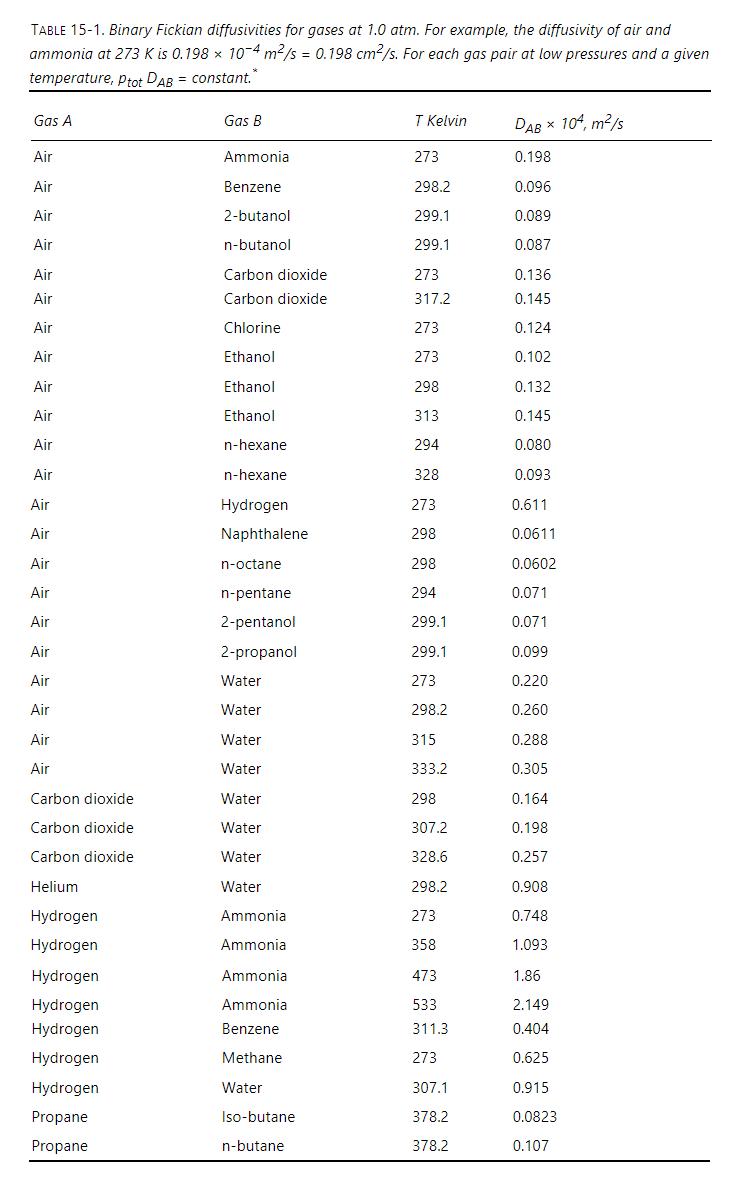
Two identical large glass bulbs are filled with gases and connected by a capillary tube that is \(\delta=0.0100 \mathrm{~m}\) long. Bulb 1 at \(\mathrm{z}=0\) contains the following mole fractions: \(\mathrm{y}_{\text {air }}=\) \(0.520, \mathrm{y}_{\mathrm{H} 2}=0.480\), and \(\mathrm{y}_{\mathrm{NH} 3}=0.000\). Bulb 2 at \(\mathrm{z}=\delta\) contains \(\mathrm{y}_{\text {air }}=0.540, \mathrm{y}_{\mathrm{H} 2}=\) 0.000 , and \(y_{\mathrm{NH} 3}=0.460\). Because the bulbs are quite large, operation is at pseudo- (or quasi-) steady state. (In other words, assume mole fractions at the boundaries are constant [e.g., \(\mathrm{y}_{\text {air }}=0.520\) at \(\mathrm{z}=0\), and \(\mathrm{y}_{\text {air }}=0.540\) at \(\mathrm{z}=\delta\) so that \(\Delta \mathrm{y}_{\text {air }}=0.02\) ] and total flux of air + hydrogen + ammonia is zero.) Pressure is uniform at \(2.00 \mathrm{~atm}\), and temperature is uniform at \(273 \mathrm{~K}\). Diffusivity values can be determined from Table 151. Assume ideal gases. Estimate fluxes of the three components using difference equation formulation of the Maxwell-Stefan method.
Table 15-1
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





