In the acrolein process introduced in problem 15.1, the catalyst is packed in tubes and the reactor
Question:
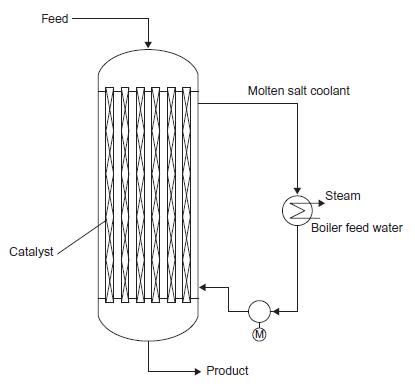
In the acrolein process introduced in problem 15.1, the catalyst is packed in tubes and the reactor is cooled using a circulating molten salt. The molten salt rejects heat to boiling steam in a similar arrangement to that shown for phthalic anhydride in Figure 15.12. The required gas hourly space velocity is 200 standard litres (at STP)/litre.h. Design and size a reactor to produce 20 kt/y of acrolein.
Data from problem 15.1
Acrolein (H2C = CHCHO) is made by selective oxidation of propylene at 2 bar, 350°C using a molybdenum, iron and bismuth catalyst on a silica support. The reactor yields based on propylene are 85% acrolein, 10% acrylic acid and 5% light byproducts. The light byproducts are mostly acetaldehyde, but for the purpose of this problem it can be assumed that the yield is 85% acrolein and 15% acrylic acid. The feed to the reactor on a volume percent basis is propylene 6%, propane 28%, steam 6%, oxygen 11%, and balance nitrogen. Estimate the reactor cooling requirement for a plant that produces 20,000 metric tons per year (20 kt/y) of acrolein if the reactor is operated isothermally.
Figure 15.12
Step by Step Answer:

Chemical Engineering Design
ISBN: 9780081025994
6th Edition
Authors: Ray Sinnott, R.K. Sinnott, Sinnott Gavin Towler





