The block diagram shows the main steps in the balanced process for the production of vinyl chloride
Question:
The block diagram shows the main steps in the balanced process for the production of vinyl chloride from ethylene. Each block represents a reactor and several other processing units. The main reactions are:
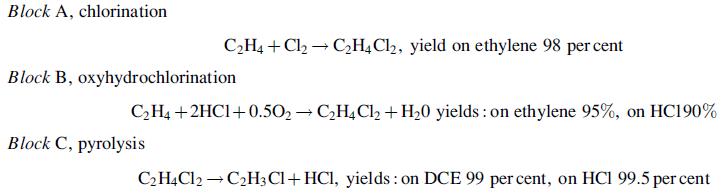
The HCl from the pyrolysis step is recycled to the oxyhydrochlorination step. The flow of ethylene to the chlorination and oxyhydrochlorination reactors is adjusted so that the production of HCl is in balance with the requirement. The conversion in the pyrolysis reactor is limited to 55%, and the unreacted dichloroethane (DCE) is separated and recycled.
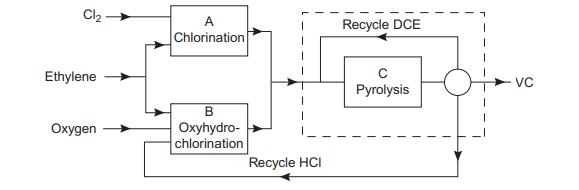
Using the yield figures given, and neglecting any other losses, calculate the flow of ethylene to each reactor and the flow of DCE to the pyrolysis reactor, for a production rate of 12,500 kg/h vinyl chloride (VC)
Step by Step Answer:

Chemical Engineering Design
ISBN: 9780081025994
6th Edition
Authors: Ray Sinnott, R.K. Sinnott, Sinnott Gavin Towler





