(a) Consider the hydrogenation of benzene to cyclohexane, which takes place by the step-by-step addition of two...
Question:
(a) Consider the hydrogenation of benzene to cyclohexane, which takes place by the step-by-step addition of two H atoms per step: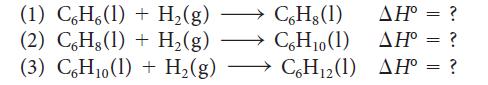
Draw Lewis structures for the products of the hydrogenation of benzene. If resonance is possible, show only one of the most important resonance structures.
(b) Use bond enthalpies to estimate the enthalpy changes of each step and the total hydrogenation. Ignore the delocalization of electrons in this calculation and the fact that the compounds are liquids.
(c) Use data from Appendix 2A to calculate the enthalpy of the complete hydrogenation of benzene to cyclohexane.
(d) Compare the value obtained in part (c) with that obtained in part (b). Explain any diff erence.
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





