Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of
Question:
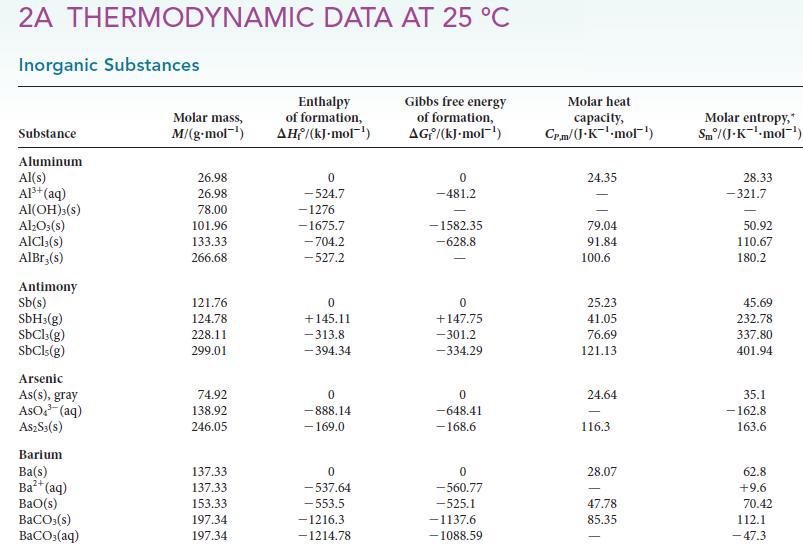
Use the standard Gibbs free energies of formation in Appendix 2A to calculate ΔG° for each of the following reactions at 25 °C. Comment on the spontaneity of each reaction under standard conditions at 25 °C.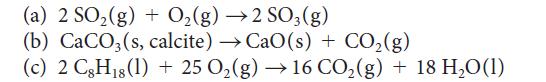
Transcribed Image Text:
(a) 2 SO₂ (g) + O₂(g) →2 SO3(g) (b) CaCO3(s, calcite) →CaO(s) + CO₂(g) (c) 2 CgHıs(l) + 25 O,(g) → 16 CO,(g) + 18 H,O(1) 18
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a AG 14174 kJ mol ...View the full answer

Answered By

John Kago
Am a processional practicing accountant with 5 years experience in practice, I also happens to have hands on experience in economic analysis and statistical research for 3 years. am well conversant with Accounting packages, sage, pastel, quick books, hansa world, etc, I have real work experience with Strata, and SPSS
4.70+
31+ Reviews
77+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Ammonia survives indefinitely in air. An agronomist studying how long ammonia survives in soil might need to know whether it survives because its oxidation is not spontaneous under ordinary...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction...
-
Consider a scenario, in which there were four students (Arthur, Kevin, Morris, and Orlando) driving their cars back home from Houston. However, only Orlando was involved in a car accident. We want to...
-
Consider the electrostatic Green functions of Section 1.10 for Dirichlet and Neumann boundary conditions on the surface S bounding the volume V. Apply Green's theorem (1.35) with integration variable...
-
In spite of the controversy about CEO Mayer's decision to ban telecommuting, she raises valid points that might affect Yahoo!'s profitability. How could each of her concerns be overcome by providing...
-
Does a man standing in an elevator that is moving upward at a constant speed of \(10 \mathrm{~m} / \mathrm{s}\) feel heavier or lighter? What if the elevator is moving downward at the same speed? If...
-
The Baxter Corporation issued $400,000 of 11% bonds for $385,279.91 on January 1, 2006. The bonds pay interest semiannually on June 30 and December 31, were issued to yield 12%, and are due on...
-
9. An arrow 2.5 cm high is placed at a distance of 25 cm from a diverging mirror of focal length 20 cm., Find the nature, position and size of the image formed. 10. The image formed by a convex...
-
(a) Consider the hydrogenation of benzene to cyclohexane, which takes place by the step-by-step addition of two H atoms per step: Draw Lewis structures for the products of the hydrogenation of...
-
Initially an ideal gas at 412 K occupies 12.62 L at 0.6789 atm. The gas is allowed to expand to 19.44 L by two pathways: (a) Isothermal, reversible expansion and (b) Isothermal, irreversible free...
-
TRW Inc. began business in 2009 and was profitable for its first three years. In 2012, it generated a $741,000 net operating loss. The following table shows TRW's taxable income before consideration...
-
Describe genba walks and explain how managers can use them to thoroughly understand situations (principle 12).
-
A commercial bank sells a Treasury bond to the Federal Reserve for $100,000. The money supply: a. increases by $100,000. b. decreases by $100,000. c. is unaffected by the transaction.
-
The Suction Business' ending inventory of vacuum cleaner parts included the following items: Required: a Calculate the value of the ending inventory under the lower-of-cost-or-net realisable value...
-
Describe three risks that are unique to each of the following two manufacturing approaches: made to order (MTO) and made to stock (MTS).
-
Suppose that last year $30 billion in new loans were extended by banks while $50 billion in old loans were paid off by borrowers. What happened to the money supply? a. Increased. b. Decreased. c....
-
Two large manufacturing firms are major sources of airborne pollutants in a metropolitan area. Currently, each firm generates about 15 million units of pollution per year. The firms costs of reducing...
-
A fast-food restaurant averages 150 customers per hour. The average processing time per customer is 90 seconds. a. Determine how many cash registers the restaurant should have if it wishes to...
-
Breeder reactors are used to convert the nonfissionable nuclide 92 235 U to a fissionable product. Neutron capture of the 92 235 U is followed by two successive beta decays. What is the final...
-
The sun radiates 3.9 10 -3 J of energy into space every second. What is the rate at which mass is lost from the sun?
-
Fresh rainwater or surface water contains enough tritium ( 3 1 H) to show 5.5 decay events per minute per 100. g water. Tritium has a half-life of 12.3 years. You are asked to check a vintage wine...
-
The distance of 2 m from point of source of sound the sun level is measured at 8 3 DB. what will be the sound level from a distance of 3 6 m from the source?
-
Problem 1. Determine the member end moments and reactions for the frames shown in Figs by using the moment-distribution method. (40pts) 1.5k/ft 20 k C 21 D E constant B 40 ft 30 fi
-
A pipeline is filled with oil traveling at a constant velocity of 4 ft/s. How long will it take for oil to reach a point 5 miles away?

Study smarter with the SolutionInn App


