(a) Explore whether the lattice energies of the alkali metal iodides are inversely proportional to the distances...
Question:
(a) Explore whether the lattice energies of the alkali metal iodides are inversely proportional to the distances between the centers of the ions in MI (M = alkali metal) by plotting the lattice energies given below against the internuclear distances d M = I.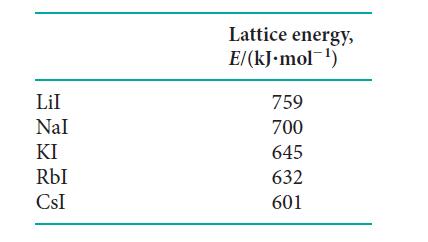
How good is the correlation? Is a better fit obtained by plotting the lattice energies against (1 - d*/d )/ d, as suggested theoretically with d* = 34.5 pm?
You should use a standard graphing program to make the plot that will generate an equation for the line and calculate a correlation coefficient for the fit.
(b) From the ionic radii given in Appendix 2D and the plot given in part (a), estimate the lattice energy of silver iodide.
(c) Compare your results from part
(b) with the experimentally determined value of 886 kJ · mol–1. If they do not agree, provide an explanation for the deviation.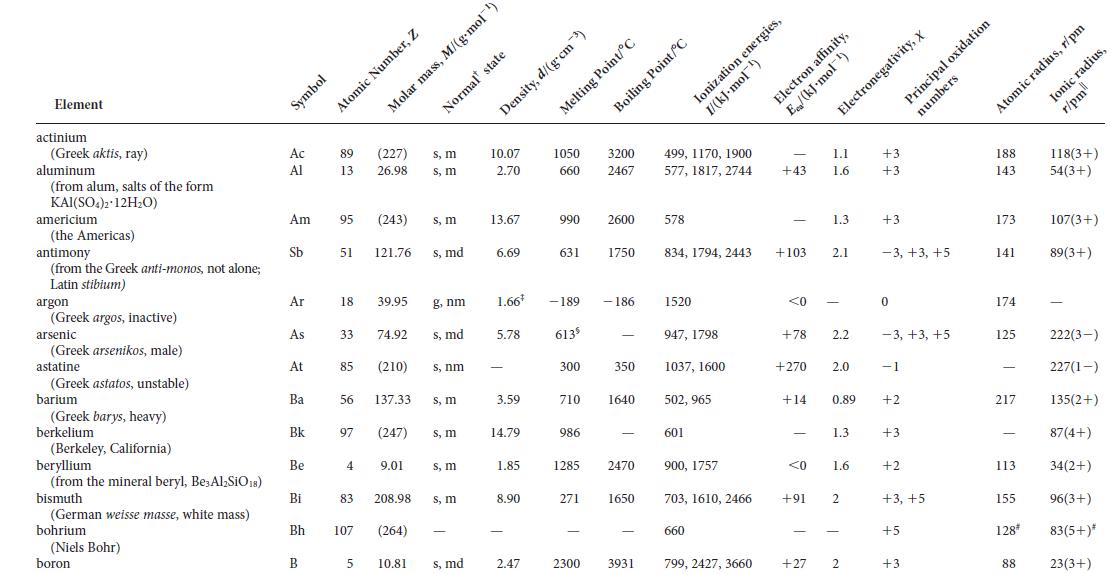
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





