A scientist proposed the following two reactions to produce ethanol, a liquid fuel: Reaction B is preferred
Question:
A scientist proposed the following two reactions to produce ethanol, a liquid fuel: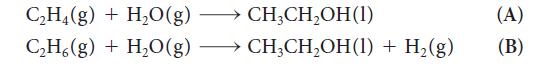
Reaction B is preferred if it is spontaneous, because C2H6(g) is a cheaper starting material than C2H4(g). Assume standard-state conditions and determine if either reaction is thermodynamically spontaneous.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





