(a) Use the Rydberg formula for atomic hydrogen to calculate the wavelength for the transition from n...
Question:
(a) Use the Rydberg formula for atomic hydrogen to calculate the wavelength for the transition from n = 3 to n = 1.
(b) What is the name given to the spectroscopic series to which this transition belongs?
(c) Use Table 1A.1 to determine the region of the spectrum in which the transition takes place.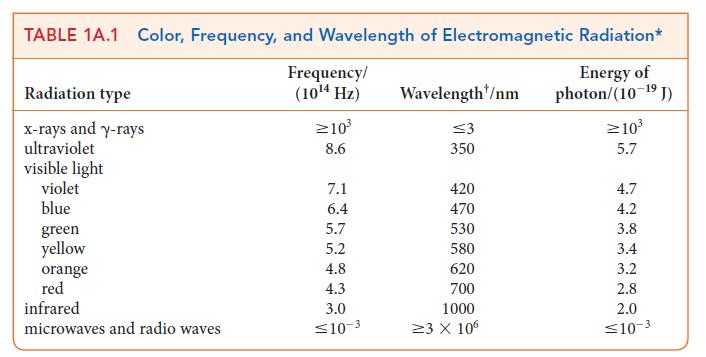
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





