Amino acids are the building blocks of proteins, which have long chainlike molecules. They are oxidized in
Question:
Amino acids are the building blocks of proteins, which have long chainlike molecules. They are oxidized in the body to urea, carbon dioxide, and liquid water. Is this reaction a source of heat for the body? Predict the standard reaction enthalpy for the oxidation of the simplest amino acid, glycine (NH2CH2COOH), a solid, to solid urea (H2NCONH2), carbon dioxide gas, and liquid water:![]()
ANTICIPATE You should expect a strongly negative value, because all combustions are exothermic and this oxidation is like an incomplete combustion.
PLAN First, add together the standard enthalpies of formation of the products, multiplying each value by the appropriate number of moles from the balanced equation. Remember that the standard enthalpy of formation of an element in its most stable form is zero. Then, calculate the total standard enthalpy of formation of the reactants in the same way and use Eq. 2 to calculate the standard reaction enthalpy.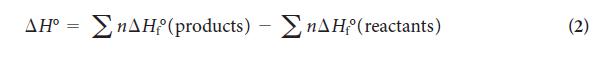
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





