An important reaction that takes place in the atmosphere is NO 2 (g) NO(g) + O(g),
Question:
An important reaction that takes place in the atmosphere is NO2(g) → NO(g) + O(g), which is brought about by sunlight. How much energy must be supplied by the Sun to cause it? Calculate the standard enthalpy of the reaction from the following information: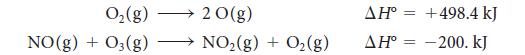
and from additional information in Appendix 2A.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





