Calculate the standard enthalpy of formation of dinitrogen pentoxide from the following data: and from the standard
Question:
Calculate the standard enthalpy of formation of dinitrogen pentoxide from the following data: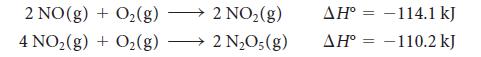
and from the standard enthalpy of formation of nitric oxide, NO.
Transcribed Image Text:
2 NO(g) + O2(g) + O2(g) 4 NO2(g) 2 NO2(g) 2 NO;(g) ΔΗ° = -114.1 kJ ΔΗ° = -110.2 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Felix Onchweri
I have enough knowledge to handle different assignments and projects in the computing world. Besides, I can handle essays in different fields such as business and history. I can also handle both short and long research issues as per the requirements of the client. I believe in early delivery of orders so that the client has enough time to go through the work before submitting it. Am indeed the best option that any client that can think about.
4.50+
5+ Reviews
19+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation of NOCI (g) from the enthalpy of formation of NO given in Table 2.5, together with the following information: 2 NOCl (g) 2 NO (g) + Clz (g) 1 Ho = + Uo=...
-
Calculate the standard enthalpy of formation (DHf) of ClO from the following bond enthalpies: Cl2: 242.7 kJ/mol; O2: 498.7 kJ/mol; ClO: 206 kJ/mol.
-
Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data at 298.15 K. Assume that the heat capacities are independent of temperature. You are also given that...
-
Prestopino Corporation produces motorcycle batteries. Prestopino turns out 1,500 batteries a day at a cost of $6 per battery for materials and labor. It takes the firm 22 days to convert raw...
-
Hindi Company has the following production data for April: units transferred out 40,000, and ending work in process 5,000 units that are 100% complete for materials and 40% complete for conversion...
-
Draw the given vectors and find their sum graphically. The magnitude is shown first, followed by the direction as an angle in standard position. 3.6 cm, 0; 4.3 cm, 90
-
A 1-kg cart and a 2-kg cart are held together with a coupler that contains a small charge. The charge is exploded and sends the \(1-\mathrm{kg}\) cart rolling away at \(+4.0 \mathrm{~m} /...
-
TJohn Phelps has run a small business for many years and has never kept adequate accounting records. However, a need to obtain a loan from the Bank of Make It Flow, for the expansion of his business...
-
3. As. Mr. Cartwright's financial advisor, would you urge him to go ahead with, or to reconsider, his anticipated expansion and his plans for additional debt financing? As the banker, would you...
-
An important reaction that takes place in the atmosphere is NO 2 (g) NO(g) + O(g), which is brought about by sunlight. How much energy must be supplied by the Sun to cause it? Calculate the standard...
-
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of the following reactions: (a) The removal of hydrogen sulfide from natural gas: (b) The...
-
Financial Paper, Inc. is a printer of checks and forms for financial institutions. For individual accounts, boxes of 200 checks cost $0.80 per box to print and package and sell for $4.95 each....
-
What are the different types of organizational plans? Are they independent of each other?
-
What are the characteristics of a multi-domestic corporation?
-
Job stress is a major problem for employees working in many organizations today. Discuss some of the job stressors. What can a manager do to reduce stressors for employees?
-
A coworker takes credit for the excellent job youve performed. Frustrating! Its probably happened to you or someone you know. How did it happen? Perhaps you shared an idea with a coworker and then...
-
Mintzberg suggests that managerial roles should encompass interpersonal, decisional, and informational roles. Clearly, this is an idealized vision of the manager. They are encouraged to encompass all...
-
True or False: 1. Government spending as a percentage of GDP has changed little since 1970, but the composition of government spending has changed considerably. 2. The composition of state and local...
-
Suppose that you are part of a virtual team and must persuade other team members on an important matter (such as switching suppliers or altering the project deadline). Assuming that you cannot visit...
-
Which of the following statements is (are) false? Explain why the starementfs) is (are) false. a. Is a structural isomer of pentonic acid. b. Is a structual isomer of 2-methyl-3-pentanone. c....
-
The following organic compounds cannot exist. Why? a. 2-chloro-2-butyne b. 2-methyl-2-propanone c. 1,1-dimethylbenzene d. 2-pentanal e. 3-hexanoic acid f. 5,5-dibromo-l-cyclobutanol
-
Mycomycin is a naturally occurring antibiotic produced by the fungus Nocardia acidophilus. The molecular formula of the substance is Q3H10O2, and its systematic name is...
-
An organization is considering adopting a new technology that could potentially replace 350 human jobs at their largest manufacturing facility. 1. What ethical considerations should be taken into...
-
Your task is to create a business proposal for a product or service w hich you believe will work in the Canadian Market. Your Outline will include the following information 1 . Summary of Idea ( The...
-
Beauty Works is a company that creates skin care products sourced from natural materials in South America from environmentally sustainable farms. You are the project manager for Beauty Works and has...

Study smarter with the SolutionInn App


