Barium metal is produced by the reaction of aluminum metal with barium oxide. From the standard reaction
Question:
Barium metal is produced by the reaction of aluminum metal with barium oxide. From the standard reaction enthalpies,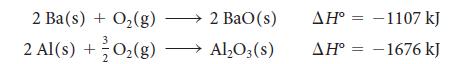
calculate the reaction enthalpy for the production of metallic barium in the reaction![]()
Transcribed Image Text:
2 Ba(s) + O₂(g) 2 Al(s) +O₂(g) 2 BaO(s) Al₂O3(s) AH° -1107 kJ AH° -1676 kJ = = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Aluminum metal reacts with iron(III) oxide to produce aluminum oxide and iron metal. a. How many moles of Fe2O3 are required to completely react with 41 g Al? b. How many moles of Fe are produced by...
-
Aluminum metal reacts with iron(III) oxide to produce aluminum oxide and iron metal. a. How many moles of Fe 2 O 3 are required to completely react with 44 g Al? b. How many moles of Fe are produced...
-
Monochlorobenzene is produced by the reaction of benzene with chlorine. A mixture of monochlorobenzene and dichlorobenzene is produced, with a small amount of trichlorobenzene. Hydrogen chloride is...
-
On January 1, 2012, Wilmes Floral supplies borrowed $2,413 from Bower Financial Services. Wilmes Floral Supplies gave Bower a $2,500 note with a maturity date of December 31, 2013. The note specified...
-
(a) Eve Adams believes the production cost report is an external report for stockholders. Is Eve correct? Explain. (b) Identify the sections in a production cost report.
-
Find the indicated vector sums and differences with the given vectors by means of diagrams. B + 3E A B C D E
-
How much energy is dissipated in the collision of Checkpoint 7.11? Data from Checkpoint 7.11 A 1000-kg compact car and a \(2000-\mathrm{kg}\) van, each traveling at \(25 \mathrm{~m} / \mathrm{s}\),...
-
Assume the United States exports 1,000 computers at a price of $3,000 each and imports 150 UK autos at a price of 10,000 each. Assume that the dollar/pound exchange rate is $2 per pound. a....
-
Assuming a rate of return of 8%, calculate the monthly savings needed for education assuming that savings will continue until the children's college education is completed. Education Because of the...
-
An ice cube of mass 50.0 g at 0.0 C is added to a glass containing 400.0 g of water at 45.0C. What is the final temperature of the system? Assume that no heat is lost to the surroundings.
-
Determine the reaction enthalpy for the hydrogenation of ethyne to ethane, C 2 H 2 (g) + 2 H 2 (g) C 2 H 6 (g), from the following data: H c (C 2 H 2 , g) = 1300. kJ mol 1 , H c (C 2 H 6 , g) =...
-
A 1500-kg blue convertible is traveling south, and a 2000-kg red SUV is traveling west. If the total momentum of the system consisting of the two cars is 7200 kg m/s directed at 60.0 west of south,...
-
The Americans with Disabilities Act requires that employers hire workers with disabilities whether or not they are otherwise qualified for the work. (True/False)
-
Deeds offer different degrees of protection against defects of title. (True/False)
-
When directors do not act in the best interests of their corporation, the shareholders may sue them on the companys behalf. (True/False)
-
Why might a bankruptcy petition be dismissed?
-
When does a lender have the right to foreclose on mortgaged property?
-
Multiple Choice Questions 1. The ability to pay principle states: a. Those with the greatest ability to pay taxes should pay more. b. Those with the least ability to pay taxes should pay more. c....
-
The following table shows the rates of total return in successive years from 2004 to 2008 for the Sprott Canadian Equity Fund and for the benchmark Toronto Stock Exchange S&P/TSX Composite Index. By...
-
Is ocranoic acid more soluble in 1 M HC1, 1 M NaOH, or pure water? Explain. Drugs such as morphine are often treated with strong acids. The most commonly used form of morphine is morphine...
-
Consider the reaction to produce the ester methyl acetate: When this reaction is carried our with CH3OH contain¬ing radioactive oxygen-18, the water produced does not contain oxygen-18. Explain...
-
A compound containing only carbon and hydrogen is 85.63% C by mass. Reaction of this compound with H2O produces a secondary alcohol as the major product and a primary alcohol as the minor product. If...
-
On May 1, Crane Company had 430 units of inventory on hand, at a cost of $4.00 each. The company uses a perpetual inventory system. All purchases and sales are on account. A record of inventory...
-
Write the expression as one logarithm. 5 loga x + 3 * loga(x 9) 7 log,(5x + 8)
-
Solve the equation for the requested variables. z(cr+x) = n + h Solve for z: z = Solve for r: r =

Study smarter with the SolutionInn App


