Monochlorobenzene is produced by the reaction of benzene with chlorine. A mixture of monochlorobenzene and dichlorobenzene is
Question:
Monochlorobenzene is produced by the reaction of benzene with chlorine. A mixture of monochlorobenzene and dichlorobenzene is produced, with a small amount of trichlorobenzene. Hydrogen chloride is produced as a byproduct. Benzene is fed to the reactor in excess to promote the production of monochlorobenzene.
The reactor products are fed to a condenser where the chlorobenzenes and unreacted benzene are condensed. The condensate is separated from the noncondensable gases in a separator. The noncondensables, hydrogen chloride and unreacted chlorine, pass to an absorption column where the hydrogen chloride is absorbed in water. The chlorine leaving the absorber is recycled to the reactor.
The liquid phase from the separator, containing chlorobenzenes and unreacted benzene, is fed to a distillation column, where the chlorobenzenes are separated from the unreacted benzene. The benzene is recycled to the reactor. Using the following data, calculate the stream flows and draw up a preliminary flowsheet for the production of 1.0 metric ton (tonne) of monochlorobenzene per day.
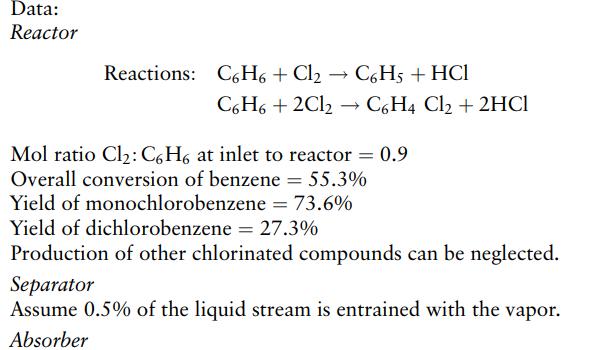
Assume 99.99% absorption of hydrogen chloride and that 98% of the chlorine is recycled, the remainder being dissolved in the water. The water supply to the absorber is set to produce a 30% w/w strength hydrochloric acid.
Distillation column Take the recovery of benzene to be 95%, and 99.99% recovery of the chlorobenzenes.
Step by Step Answer:





