Below is the titration curve for the neutralization of 25 mL of a monoprotic acid with a
Question:
Below is the titration curve for the neutralization of 25 mL of a monoprotic acid with a strong base. Answer the following questions about the reaction and explain your reasoning in each case.
(a) Is the acid strong or weak?
(b) What is the initial hydronium ion concentration of the acid?
(c) What is Ka for the acid?
(d) What is the initial concentration of the acid?
(e) What is the concentration of base in the titrant?
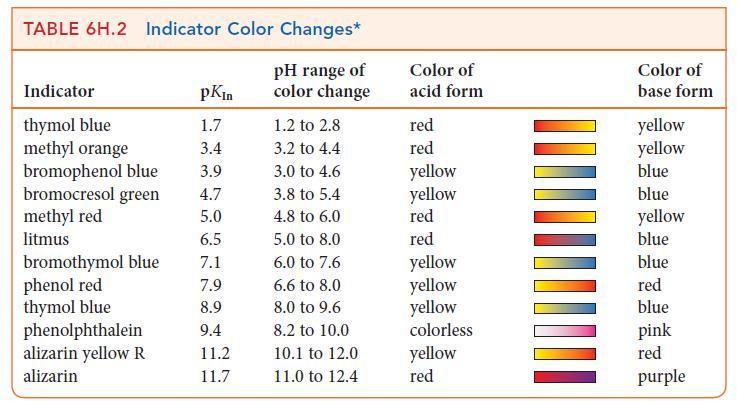
(f) Use Table 6H.2 to select an indicator for the titration.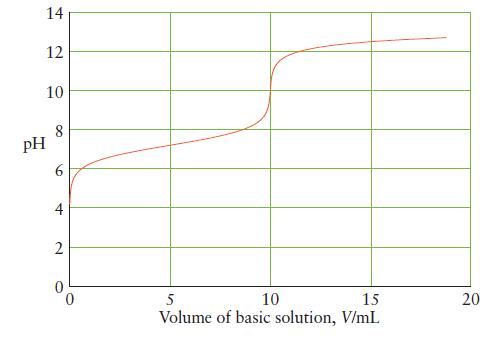
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





