Suppose the reference electrode for Table 6M.1 were the standard calomel electrode, Hg 2 Cl 2 /Hg,
Question:
Suppose the reference electrode for Table 6M.1 were the standard calomel electrode, Hg2Cl2/Hg, Cl2([Cl2] = 1.00 mol · L–1), with its E° set equal to 0. Under this system, what would be the potential for
(a) The standard hydrogen electrode;
(b) The standard Cu2+/Cu redox couple?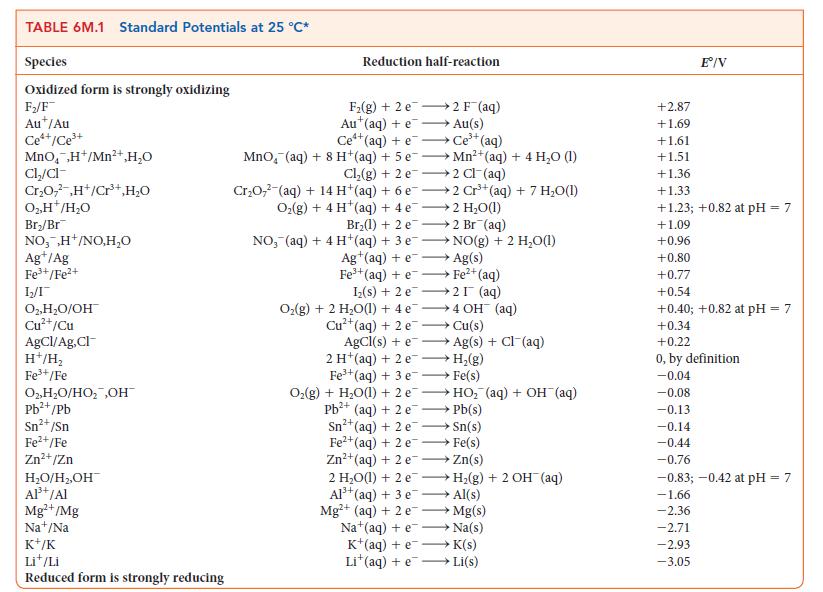
Transcribed Image Text:
TABLE 6M.1 Standard Potentials at 25 °C* Species Oxidized form is strongly oxidizing F₂/F Aut/Au Ce*+/Ce³+ MnO₂ ,H+/Mn²+,H₂O Cl₂/cl Cr₂O,²,H+/Cr³+,H₂O O₂, H*/H₂O Br₂/Br NO,,H+/NO,H₂O Ag+/Ag Fe³+/Fe²+ 1₂2/1 O2, H₂O/OH Cu²+/Cu AgCl/Ag,Cl- H+/H₂ Fe³+/Fe O₂, H₂O/HO₂ ,OH™ Pb²+/Pb Sn²+ /Sn Fe²+/Fe Zn²+/Zn H₂O/H₂,OH™ Al³+ /Al Mg²+/Mg Nat/Na K+/K Lit/Li Reduced form is strongly reducing Reduction half-reaction F₂(g) +2 e Aut(aq) +e Ce¹+(aq) +e Ce³+ (aq) MnO, (aq) + 8 H+(aq) + 5 eMn²+ (aq) + 4 H₂O (1) Cl₂(g) + 2e →2 Cl(aq) Cr₂O7² (aq) + 14 H+ (aq) + 6 e2 Cr³+ (aq) + 7 H₂O(1) Oz(g)+4H*(aq)+4e —+2H,O(l) 2 F¯ (aq) Au(s) Br₂(1) + 2 e2 Br (aq) NO, (aq) + 4H+(aq) + 3 e Ag+ (aq) + e Fe³+ (aq) +e→→→ Fe²+ (aq) AgCl (s) + e 2 H (aq) + 2 e Fe³+ (aq) + 3 e NO(g) + 2 H₂O(1) Ag(s) Iz(s) + 2 e 2 (aq) O₂(g) + 2 H₂O(1) + 4e4 OH (aq) →→→ Cu(s) 2+ Cu²+ (aq) + 2 e Fe²+(aq) + 2 e Zn²+ (aq) + 2 e →→→ Ag(s) + Cl¯(aq) H₂(g) Fe(s) O₂(g) + H₂O(1) + 2 eHO₂ (aq) + OH¯ (aq) Pb²+(aq) + 2e →→→→ Pb(s) Sn²+ (aq) + 2e →→→→Sn(s) →→→→Fe(s) Zn(s) 2 H₂O(l) + 2 eH₂(g) + 2 OH(aq) Al³+ (aq) + 3 e- →→→→ Al(s) Mg²+ (aq) + 2 e→→→→→→ Mg(s) Na (aq) + e→→→→→→→ Na(s) K+(aq) +e →→→ K(s) Lit(aq) +eLi(s) +2.87 +1.69 +1.61 +1.51 Eº/V +1.36 +1.33 +1.23; +0.82 at pH = 7 +1.09 +0.96 +0.80 +0.77 +0.54 +0.40; +0.82 at pH = 7 +0.34 +0.22 0, by definition -0.04 -0.08 -0.13 -0.14 -0.44 -0.76 -0.83; -0.42 at pH = 7 -1.66 -2.36 -2.71 -2.93 -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 0...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Consider the question posed in Exercise 6N.19 except that a saturated calomel electrode (the solution is saturated with KCl instead of having [Cl ] = 1.00 mol L 1 ) is used in place of the standard...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Smart housing Inc. is negotiating a deal to build a house. The owner wants to start in early spring when the weather begins to moderate and build through the summer into the fall. The completion time...
-
A plane, harmonic, acoustical wave that oscillates in air with an amplitude of 10-6 m has an intensity of 10-2 W/m2. What is the frequency of the sound wave?
-
Table depicts the supply and demand schedules of gloves for Portugal, a small nation that is unable to affect the world price. On graph paper, draw the supply and demand schedules for gloves in...
-
The probability that a wafer contains a large particle of contamination is 0.01. If it is assumed that the wafers are independent, what is the probability that exactly 125 wafers need to be analyzed...
-
Southwest Gift Shop, a retail business, started business on April 29, 2016. It keeps a $300 change fund in its cash register. The cash receipts for the period from April 29 to April 30, 2016, are...
-
Insight Bank has a $ 14 million position in a ten-year, zero-coupon bond with a face value of $17 000 000. The bond is trading at a yield to maturity of 6.7 per cent. The historical mean change in...
-
Warf Computers, Inc., was founded 15 years ago by Nick Warf, a computer programmer. The small initial investment to start the company was made by Nick and his friends. Over the years, this same group...
-
Calculate the molar concentration of OH in solutions with the following molar concentrations of H 3 O + : (a) 0.020 mol L 1 ; (b) 1.0 * 10 5 mol L 1 ; (c) 3.1 mol L 1 .
-
Below is the titration curve for the neutralization of 25 mL of a monoprotic acid with a strong base. Answer the following questions about the reaction and explain your reasoning in each case. (a) Is...
-
What is the purpose of a subtype discriminator?
-
Let \(f\) be a (bounded) function. Prove that \[\lim _{t ightarrow \infty} \sqrt{t} \mathbb{E}\left(f\left(M_{t} ight) \mid \mathcal{F}_{s} ight)=c\left(f\left(M_{s} ight)\left(M_{s}-W_{s}...
-
Let \(a\) and \(\sigma\) be continuous deterministic functions, \(B\) a BM and \(X\) the solution of \(d X_{t}=a(t) X_{t} d t+\sigma(t) d B_{t}, X_{0}=x\). Let \(T_{0}=\inf \left\{t \geq 0, X_{t}...
-
Let \(H\) be a measurable map defined on \(\mathbb{R}^{+} \times \Omega \times \mathbb{R}\). Prove that, for any random time \(\tau\), \[\int_{0}^{\tau} H\left(s, \omega, B_{s} ight) d...
-
Indicate whether each of the following statements is true or false by writing T or F in the answer c olumn. The writing a holder puts on the back of a negotiable instrument is known as a negotiation.
-
If the governments budget is a deficit of $1 trillion, what are the real interest rate and investment? Does any crowding out occur? Problem 8 The table sets out the data for an economy when the...
-
A random sample of 51 items is taken, with = 58.42 and s2 = 25.68. Use these data to test the following hypotheses, assuming you want to take only a 1% risk of committing a Type I error and that x...
-
Study the pictures/images below. Obviously these was focus on LT sociology, anthropology and poltical science. Try to do some analysis by finding clues that are synonymous with the main concepts....
-
All of the following acid-base reactions are reactions that we will study in greater detail in the chapters to follow. For each one, draw the mechanism and then clearly label the acid, base,...
-
Each of the following mechanisms contains one or more errorsthat is, the curved arrows may or may not be correct. In each case, identify the errors and then describe what modification would be...
-
In an intramolecular proton transfer reaction, the acidic site and the basic site are tethered to the same molecule, and a proton is passed from the acidic region of the molecule to the basic region...
-
Antoine pays the following amounts in the current year: $100 vehicle inspection tax $200 annual toll charge for bridge $300 sewer and water assessment $400 real estate assessment for street...
-
Give an example of Starbucks company how they integrate the Enterprise Resource Planning (ERP), Supply Chain Management Software (SCM), Customer Relationship Management (CRM), and Transaction...
-
Evaluate the integral. 4x+5 So 2x2+5x+1 dx

Study smarter with the SolutionInn App


