By assuming that the lattice enthalpy of NaCl 2 is the same as that of MgCl 2
Question:
By assuming that the lattice enthalpy of NaCl2 is the same as that of MgCl2, use enthalpy arguments based on data in Appendix 2A, explain why NaCl2 is an unlikely compound.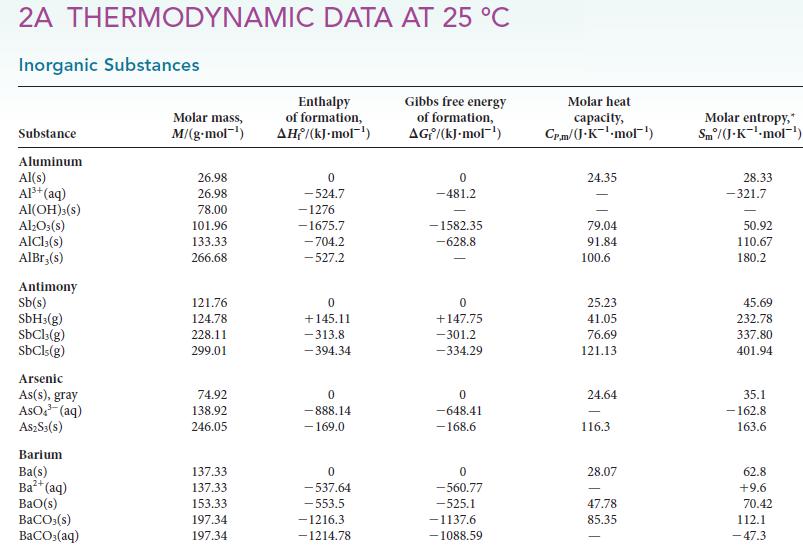
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) Al(OH)3(S) Al₂O3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO4³ (aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-¹) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy,* Sm/(J-K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
In order to assess the likelihood of the existence of NaCl by assuming that its lattice enthalpy is ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Based on data in Table 8.2, estimate (within 30 kJ / mol) the lattice energy for (a) LiBr, (b) CsBr, (c) CaCl2. TABLE 8.2 Lattice Energies for Some Ionic Compounds Lattice Energy (kJ/mol) Lattice...
-
In Problem 15-48, you were asked to use best subsets stepwise regression to establish the relationship between body fat and the independent variables weight, abdomen circumference, and thigh...
-
The lattice enthalpy of sodium chloride, H for NaCl(s) Na+(g) + Cl(g)
-
ABC Company produces and sells I product. Once the products are produced, they are sold, and there is no work-in- process, no any inventory in stock. Company uses standard costing method in its...
-
The net income reported on the income statement for the current year was $132,000. Depreciation recorded on store equipment for the year amounted to $21,800. Balances of the current asset and current...
-
Each quadratic function has the form y = ax 2 + bx + c. Identify a, b, and c. y = 3x 2 - 4x
-
What reasons might an entity provide for adopting ESG practices?
-
Solve the model formulated in Problem 7 for Southern Sporting Goods Company graphically. a. Identify the amount of unused resources (i.e., slack) at each of the graphical extreme points. b. What...
-
Step 3: Testing Your Configuration Update 1. Restart the SSH service. [Your solution command here] 2. Exit the root account. [Your solution command here] 3. SSH to the target machine using your sysd...
-
The production of steel from iron ore is endothermic. To reduce the heat that must be supplied, engineers need to find the lowest temperature at which the desired reactions are spontaneous. Estimate...
-
The standard entropy of vaporization of acetone is approximately 85 J K 1 mol 1 at its boiling point. (a) Estimate the standard enthalpy of vaporization of acetone at its boiling point of 56.2 C....
-
Use sum, difference, product, or half-angle formulas to find the exact value of the expression. tan75
-
Define the term payback period. Why is the payback period a poor measure for determining whether a capital expenditure proposal is acceptable?
-
Develop a list of the sales tools that the salesperson should consider when planning a sales demonstration.
-
List the common types of buyer concerns that might surface in a presentation.
-
The trial balance of Halsey Architectural Consultants on 30 June of the current year (the end of its annual accounting period) included the following account balances before adjustments. In reviewing...
-
Develop a list of sales tools you could use in a job interview. What tools could you use to demonstrate your skills and capabilities?
-
Firms M and N compete for a market and must independently decide how much to advertise. Each can spend either $10 million or $20 million on advertising. If the firms spend equal amounts, they split...
-
You are planning to purchase your first home five years from today. The required down payment will be $50,000. You currently have $20,000. but you plan to contribute $500 each quarter to a special...
-
Chromium compounds exhibit a variety of bright colors. When solid ammonium dichromate, (NH 4 ) 2 Cr 2 O 7 , a vivid orange compound, is ignited, a spectacular reaction occurs, as shown in the two...
-
Caffeine, a stimulant found in coffee, tea, chocolate, and some medications, contains 49.48% carbon, 5.15% hydrogen, 28.87% nitrogen, and 16.49% oxygen by mass and has a molar mass of 194.2....
-
A white powder is analyzed and found to contain 43.64% phosphorus and 56.36% oxygen by mass. The compound has a molar mass of 283.88g. What are the compounds empirical and molecular formulas?
-
The Martin-Rehak Institution recently released a successful line of baseball jerseys and is seeking to begin issuing dividends. The first dividend of $1.50 will be paid 4 years from now. Dividends...
-
You are promised a cash flow every year forever! Next year you will receive $100. After that, the payment will grow by 1% compounded annually (i.e., to 100 * 1.01 in Year 2, 100 * 1.01^2 in Year 3,...
-
You have $500,000 in a bank account earning 6.50%, compounded annually. For how many years can you withdraw $40,000 at the beginning of the year before the balance is reduced to zero?

Study smarter with the SolutionInn App


