Calculate (Delta S_{text {surr }}) for the following reactions at (25^{circ} mathrm{C}) and (1 mathrm{~atm}). a. C3H8(g)
Question:
Calculate \(\Delta S_{\text {surr }}\) for the following reactions at \(25^{\circ} \mathrm{C}\) and \(1 \mathrm{~atm}\).
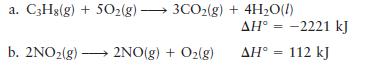
Transcribed Image Text:
a. C3H8(g) + 5O(g) 3CO2(g) + 4HO(l) b. 2NO2(g) 2NO(g) + O(g) AH = -2221 kJ AH = 112 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
The change in entropy of the surroundings Delta Stextsurr for a reaction can be determined by lookin...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate DG and Kc for the following reactions at 25C: (a) (b) (c) (d) 2 + Br2() 21 (aq)2Br (aq) I2(s) 02(8) 4H (a) 4Fe (aq) 2H20(1) + 4Fe (aq) 2.A l (s) + 312(s) 2A13+(aq) + 6(aq)
-
Calculate ÎSsurr for the following reactions at 25oC and 1 atm. a. C3Hsg) 502(g)3CO2ig) 4H20(D -2221 kJ ' '-112 kJ b, 2N02(g)-2N0(g) + O2(g)
-
The equilibrium constant of the reaction CO + ½ O2 CO2 at 1000 K and 1 atm is KP1 Express the equilibrium constant of the following reactions at 1000 K in terms of KP1: (a CO10CO at 3 atm at 1...
-
In your hometown what system is used to price the publicly supplied water? Why was that pricing system chosen? Would you recommend an alternative?
-
Consider the Henon map described by Let a = 1.4 and b = 0.3, and use a computer to plot the first 10,000 points (xw yn) starting from the initial values x0 = 0, y0 =0. Choose the plot region as 1.5 -...
-
(a) Assuming an 8 percent sales tax levied on all consumption, complete the following table: (b) Is the sales tax (A) progressive or (B) regressive? Percentage of Income Paid in Taxes Consumption...
-
Which of the following is not a control activity? a. Mandatory vacations b. Risk assessment C. Security measures d. Proper authorization
-
Foot Locker Inc. is the worlds number one retailer of athletic footwear and apparel. Headquartered in New York City, the company has over 44,000 employees and 3369 retail stores in 23 countries...
-
What role do organizational learning mechanisms, such as single-loop and double-loop learning, play in facilitating adaptive responses to environmental changes and competitive pressures ?
-
Given the following data: calculate \(\Delta G^{\circ}\) for the reaction \[6 \mathrm{C}(s)+3 \mathrm{H}_{2}(g) \longrightarrow \mathrm{C}_{6} \mathrm{H}_{6}(l)\] 2C6H6(l) +150(g) 12CO(g) + 6HO(l)...
-
Choose the compound with the greatest positional probability in each case. a. \(1 \mathrm{~mol}_{\text {of }} \mathrm{H}_{2}\) at STP or \(1 \mathrm{~mol}_{\text {of }} \mathrm{H}_{2}\) at...
-
Compare auditors common law liability to clients and third-party beneficiaries with their common law liability to other third parties.
-
Reflection on A Real-Life Executive Compensation Scheme: Please skim through the articles below, you can refer to other academic/reliable articles:...
-
1. Two firms bid for a contract to build a university building. Their construction costs are independent and uniformly drawn from [0, 1]. Both bidders submit their bids si- multaneously. The winner...
-
What roles do the primary and second markets play in the current economy? What is the relationship between financial markets and financial institutions? How could the relationship run smoother?
-
The expected dividends for the next 3 years are given: D 1 = 1 . 8 , D 2 = 2 , D 3 = 3 . from the third year, the dvidends are expected to grow at a constant rate of % 8 . If the discount rate is % 4...
-
If a uniform pressure of 120 kPa is applied at the surface of the soil, what would be the total final A soil layer at a site consisting of 6 m clay over bedrock? The water table is 1m below the...
-
Divide into two teams. One team must prepare a presentation advocating for the instrumental model of corporate management. The other team must prepare a presentation arguing for the social contract...
-
The nitrogen atoms in N2 participate in multiple bonding, whereas those in hydrazine, N2H4, do not. (a) Draw Lewis structures for both molecules. (b) What is the hybridization of the nitrogen atoms...
-
The rate law of the reaction 2 NO(g) + 2 H 2 (g) N 2 (g) + 2 H 2 O(g) is Rate = kr[NO] 2 [H 2 ], and the mechanism that has been proposed is (a) Which step in the mechanism is likely to be rate...
-
(a) Use a graphing calculator or graphing software to calculate the activation energy for the acid hydrolysis of sucrose to give glucose and fructose (see Exercise 7.9) from an Arrhenius plot of the...
-
The decomposition of A has the rate law Rate = k r [A] a . Show that for this reaction the ratio t 1/2 /t 3/4 , where t 1/2 is the half-life and t 3/4 is the time for the concentration of A to...
-
Thayer Farm trust made a farmer a loan of $1, 200 at 16% for three years compounded annually. Find the future value and the compound interest paid on the loan. Compare the compound interest with...
-
What types of questions would you ask employees and other managers as you conduct a product performance analysis? Provide two examples and explain why answers to these questions would aid in your...
-
There are three brief articles below that address both sides of the issue and a fourth attachment that presents Home Depot's statement on their approach to corporate responsibility from the 2022...

Study smarter with the SolutionInn App


