Given the following data: calculate (Delta G^{circ}) for the reaction [6 mathrm{C}(s)+3 mathrm{H}_{2}(g) longrightarrow mathrm{C}_{6} mathrm{H}_{6}(l)] 2C6H6(l)
Question:
Given the following data:
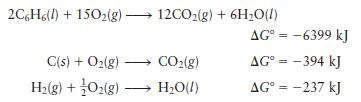
calculate \(\Delta G^{\circ}\) for the reaction
\[6 \mathrm{C}(s)+3 \mathrm{H}_{2}(g) \longrightarrow \mathrm{C}_{6} \mathrm{H}_{6}(l)\]
Transcribed Image Text:
2C6H6(l) +150(g) 12CO(g) + 6HO(l) C(s) + O(g) H(g) + O2(g) - CO(g) HO(l) AG = -6399 kJ AG = -394 kJ AG = -237 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the standard Gibbs free energy change Delta Gcirc for the target reaction 6 textCs 3 textH2g ightarrow textC6textH6l We need to use the s...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
You are a financial analyt in an investment advisory firm and you are helping your client to evaluate the value of a potential M&A target, WWG, inc. You estimate that WWG's enterprise value is $457.6...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Suppose you come from a part of the world that is blessed with abundant water. Demand never comes close to the available amount. Should you be careful about the amount you use or should you simply...
-
A circuit with a nonlinear inductor can be modeled by the first-order differential equations. Chaotic oscillations for this situation have been extensively studied. Use a computer to construct the...
-
For the water tank shown in Fig. 4.43, compute the magnitude and location of the total force on the inclined wall. 8 ft Water 60 15 ft 10 ft
-
Stone Brewing Co. is a San Diego brewer that has sold its beers for over two decades. Stone has maintained its trademark and brand from the beginning, registering the STONE mark in 1998. Stone has...
-
John, Jake, and Joe are partners with capital accounts of $90,000, $78,000, and $64,000 respectively. They share profits and losses in the ratio of 30:40:30. When the partners decide to liquidate,...
-
Unpolarized sunlight goes through two pairs of sunglasses. The second pair is oriented 20 o respect to the first. What is the exiting intensity in terms of the initial intensity I0?
-
For each of the following pairs, choose the substance with the higher positional probability (per mole) at a given temperature. a. solid CO 2 and gaseous CO 2 b. N 2 gas at 1 atm and N 2 gas at 1.0 ...
-
Calculate \(\Delta S_{\text {surr }}\) for the following reactions at \(25^{\circ} \mathrm{C}\) and \(1 \mathrm{~atm}\). a. C3H8(g) + 5O(g) 3CO2(g) + 4HO(l) b. 2NO2(g) 2NO(g) + O(g) AH = -2221 kJ AH...
-
A sample of n = 18 observations from population A has a sample standard deviation of sx = 6.48, and a sample of m = 21 observations from population B has a sample standard deviation of sy = 9.62....
-
If an agile development team has already been doing testing during each sprint, why should they consider using parallel independent testing? 1. Software developers are not trained to perform tests 2....
-
If the government expects that a substantial portion of the resources supporting a special revenue fund's activities will no longer be derived from restricted and committed revenue sources, the...
-
Explain five (5) steps on how to develop an Internal Factor Evaluation (IFE) Matrix. Provide example for each step.
-
A company has issues with users deleting general ledger accounts that were created for future posting group usage but have nothing posted to them. You need to prevent deletion of these general ledger...
-
What is project management? What is the definition of a project? What are the basic components of project management? What is the role of a project manager? Can you describe these topics in the...
-
Fill in the missing items for the followinginventories: $51,000 48,000 $28,400 24,800 88,000 - $.67,000 56,000 Beginning balance Ending balance .. Transferred in Transferred out. 57,000 170,000
-
The column shown in the figure is fixed at the base and free at the upper end. A compressive load P acts at the top of the column with an eccentricity e from the axis of the column. Beginning with...
-
The following mechanism has been proposed for the formation of hydrazine in the overall reaction, N 2 (g) + 2 H 2 (g) N 2 H 4 (g): The rate law for the overall reaction is Rate = kr[N 2 ][H 2 ] 2 ....
-
The hydrolysis of sucrose (C 12 H 22 O 11 ) produces fructose and glucose: C 12 H 22 O 11 (aq) + H 2 O(l) C 6 H 12 O 6 (glucose, aq) + C 6 H 12 O 6 (fructose, aq). Two mechanisms are proposed for...
-
Some organic compounds containing the C=O group can react with themselves in a process known as aldol condensation. The mechanism for this reaction in acidic solution is shown here. Write the overall...
-
You learned about Volkswagen's ethical dilemma when it developed the so - called clean diesel engine. For your discussion this week, select a company who had a similar ethical situation and address...
-
Test marketing is a step in the new product development process. In the chapter it was stated that some marketers see test marketing as an essential step, almost a mandatory step. Other marketers see...
-
What does it take to create completely new innovative organizations, and what are some examples of these?

Study smarter with the SolutionInn App


