Calculate the reaction enthalpy for the synthesis of hydrogen bromide gas, H 2 (g) + Br 2
Question:
Calculate the reaction enthalpy for the synthesis of hydrogen bromide gas, H2(g) + Br2(l) → 2 HBr(g), from the following data: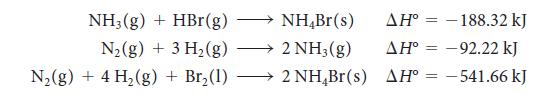
Transcribed Image Text:
NH3(g) + HBr (g) N₂(g) + 3 H₂(g) N₂(g) + 4 H₂(g) + Br₂ (1) NH,Br(s) 2NH,(g) → 2 NH₂Br(s) AH-188.32 kJ -92.22 kJ AH-541.66 kJ AH° =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The gas-phase reaction between Br2 and H2 to form HBr is assumed to proceed by the following mechanism: a. Under what conditions does the rate law have the form rate = k[Br2]? b. Under what...
-
The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.80. (a) Calculate the overall standard enthalpy change for the reaction process. (b) HBr does not react with...
-
Calculate the reaction enthalpy for the formation of anhydrous aluminum bromide, 2 Al(s) + 3 Br 2 (l) 2 AlBr 3 (s), from the following data: 2 Al(s) + 6 HBr(aq) - HBr(g) H(g) + Br (1) AlBr3 (s) 2...
-
George bought the following amounts of Stock A over the years: (Loss amounts should be indicated with a minus sign.) Number of Adjusted Basis $ 7,200 6,270 Date Purchased Shares Stock A 11/21/1993...
-
Bristol Company identifies three activities in its manufacturing process: machine setups, machining, and inspections. Estimated annual overhead cost for each activity is $120,000, $300,000, and...
-
Two brewing companies that compete against each other are Northern Ale Ltd. (NAL) and Brew Right Inc. (BRI). The industry is experiencing a slump and each company has undertaken a different strategy...
-
If you drop a book from a certain height, it falls (accelerating all the while) because of the gravitational force exerted by Earth on it. Because forces always come in interaction pairs, the book...
-
Sarah Beth's Art Supply Company produces various types of paints. Actual direct manufacturing labor hours in the factory that produces paint have been higher than budgeted hours for the last few...
-
Exercise 1 Motion on a Ramp In this exercise you will roll a marble down a ramp and calculate its linear acceleration and velocity. You will then create graphs to illustrate the motion of the marble.
-
The amino acid glutamine (Gln) is produced in the body in an enzyme-catalyzed reaction of the amino acid glutamate (Glu) with the ammonium ion: Calculate the enthalpy of this reaction at 80.0C by...
-
Calculate the standard entropy of vaporization of ammonia at 210.0 K, given that the molar heat capacities at constant pressure of liquid ammonia and ammonia vapor are 80.8 J K 1 mol 1 and 35.1 J ...
-
The clients brother was killed in an automobile collision. Family members disagree about whether viewing of the deceased should be allowed, due to the degree of damage to the body. What section of...
-
Match the following normalized impedances with points A, B, C, D, and E on the Smith chart of Figure 11.44. (i) 0 + j0 (ii) 1+ j0 (iii) 0 j1 (iv) 0 + j1 (v) + j (vi) [Zin / Z]min (vii) [Zin / Z]max...
-
Well, its my job that brought us here in the first place I am going to have to make a decision to stick with this assignment and hope I can work things out, or to return to the United States and...
-
The success of Japans Olympus was based largely on the corporate leadership system consensus driven, government supported, and rife with cronyismthat was successful when Japan was one of the...
-
Choose a company following a conglomerate diversification corporate strategy, such as Berkshire Hathaway, Reliance Industries, or Alibaba. Identify the extent to which its diversification strategy...
-
The above statements truly reflect HSBC Holdings (hereafter HSBC) massive and distinct operations in 2012 that maintained 89 million customers worldwide. Originally known as Hong Kong Shanghai...
-
Why can we not say that two people who chose to buy the same quantity of a good at the same price have the same marginal utility?
-
What is master production scheduling and how is it done?
-
Draw all the structural and geometric (cis-trans) isomers of C4H7F.
-
Draw all the structural isomers of C5H10. Ignore any cyclic isomers. Which of the structural isomers exhibit cis-trans isomerism?
-
If one hydrogen in a hydrocarbon is replaced by a halogen atom, the number of isomers that exist for the substituted compound depends on the number of types of hydrogen in the original hydrocarbon....
-
What was a central argument of the selection from Michael Foucault's book, Discipline and Punish, that we read for class?
-
1. Increases in carbon dioxide in the earth's atmosphere have been cited as a possible cause of 'global warming.' Let y represent the stock of CO2 and let x>0 (a constant) represent the flow of CO2...
-
Find (I - M) 1 and X. 0.4 0.1 0.1 14 M= 0.1 0.5 0.2 D = 16 0.1 0.1 0.5 39 Select the correct choice below and, if necessary, fill in the answer box to complete your choice. A. (I-M)1 (Type an integer...

Study smarter with the SolutionInn App


