Calculate the reaction enthalpy for the formation of anhydrous aluminum bromide, 2 Al(s) + 3 Br 2
Question:
Calculate the reaction enthalpy for the formation of anhydrous aluminum bromide, 2 Al(s) + 3 Br2(l) → 2 AlBr3(s), from the following data: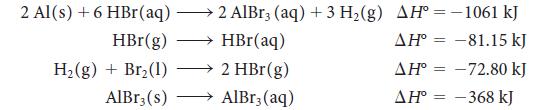
Transcribed Image Text:
2 Al(s) + 6 HBr(aq) - HBr(g) H₂(g) + Br₂ (1) AlBr3 (s) 2 AlBr3 (aq) + 3 H₂(g) AH-1061 kJ HBr(aq) AH° -81.15 kJ 2 HBr(g) AH° -72.80 kJ AlBr3(aq) AH-368 kJ = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
We need to manipulate and combine these two equations to obtain the desired reaction equation ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Calculate the reaction enthalpy for the synthesis of hydrogen bromide gas, H 2 (g) + Br 2 (l) 2 HBr(g), from the following data: NH3(g) + HBr (g) N(g) + 3 H(g) N(g) + 4 H(g) + Br (1) NH,Br(s)...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Barium metal is produced by the reaction of aluminum metal with barium oxide. From the standard reaction enthalpies, calculate the reaction enthalpy for the production of metallic barium in the...
-
QUESTION 17 The moment of a force about a pivot point is; a. The force multiplied by the perpendicular distance fro the pivot point to the direction of the force b. the distance from the pivot to the...
-
Bowyer Manufacturing Company has the following production data for selected months. Compute the physical units for eachmonth. Ending Work in Process % Complete as to Conversion Cost Beginning Work in...
-
Draw the given vectors and find their sum graphically. The magnitude is shown first, followed by the direction as an angle in standard position. 7.5 cm, 240; 2.3 cm, 30
-
While you are standing on your balcony \(8 \mathrm{~m}\) above the ground, your friend tosses a \(0.4-\mathrm{kg}\) book at you from the ground at \(14 \mathrm{~m} / \mathrm{s}\). The book barely...
-
1. Which of the following is an example of moral hazard? a. Reckless drivers are the ones most likely to buy automobile insurance. b. Retail stores located in high-crime areas tend to buy theft...
-
calculate both intrinsic value and time value for the options that you have picked. ANZ GROUP HOLDINGS LIMITED ANZ LAST PRICE / TODAY'S CHANGE $25.280+$0.350 (1.403 %) Industry Group: Banks VOLUME...
-
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of the following reactions: (a) The final stage in the production of nitric acid: (b) The...
-
Calculate the standard entropy of vaporization of water at 85C, given that its standard entropy of vaporization at 100.C is 109.0 J K 1 mol 1 and the molar heat capacities at constant pressure of...
-
Use truth tables to determine whether the following statement forms are tautologous, self-contradictory, or contingent. (p p) (q ~q)
-
Though it might be a challenge, employing individuals with autism is beneficial. According to Britains National Autistic Society, only 15 percent of adults with autism have fulltime employment, while...
-
Thomas Lopez, a lifeguard in the Miami area, was fired for leaving his assigned area to save a drowning man. His employer, Jeff Ellis and Associates, which has a contract with the Florida city of...
-
In India, only about 20 cities out of 87 have organized transport and fewer can lay claim to a mass rapid transit system. A collaboration between Indias Tata Motors and Brazils Marcopolo, it...
-
After a meeting at Creed & Dibbs CPA, Eduardo inadvertently leaves his smartphone in the firms conference room. With respect to the smartphone, this is a. an involuntary bailment. b. not a bailment...
-
Hector sells his Microsoft Xbox to Paul and Amy. Each takes a one-half interest in it. Paul and Amy are not married. Nothing is said about the form of the buyers ownership. They own the system as a....
-
Why does the creation of a government program create a special interest group, which makes it difficult to reduce or eliminate it in the future?
-
Players A, B, and C toss a fair coin in order. The first to throw a head wins. What are their respective chances of winning?
-
Oxidation of an aldehyde yields a carboxylic acid: Draw the structures for the products of the following oxidation reactions. a. b. c. [ox] propanal 2,3-dimethylpentanal ox] 3-ethylbenzaldehyde>
-
Three different organic compounds have the formula C3H8O. Only two of these isomers react with KMnO4 (a strong oxidizing agent). What are the names of the products when these isomers react with...
-
Give an example reaction that would yield the following products as major organic products. For oxidation reactions, just write oxidation over the arrow and dont worry about the actual reagent. a....
-
1. The characteristics of well written learning objectives include all of these except: Measurable standardsA Specific resources the learner needsB A statement of what the trainer will doC Conditions...
-
( i ) Assume that Operations will be able to ensure that all buffer sizes are the same as they were last year for all three options ( status 2 0 buffer continues at two weeks ) . Which of the three...
-
Entrusted with a leadership role, a manager is responsible for overseeing a department or group of employees within a specific organization.Managers are utilized in every sector, and the business...

Study smarter with the SolutionInn App


