Calculate the standard Gibbs free energy for each of the following reactions: 2 HI(g), K = 54
Question:
Calculate the standard Gibbs free energy for each of the following reactions: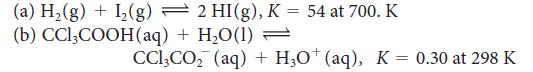
Transcribed Image Text:
2 HI(g), K = 54 at 700. K (a) H₂(g) + 1₂(g) (b) CCl3COOH (aq) + H₂O(1) = CCl₂CO₂ (aq) + H3O+ (aq), K = 0.30 at 298 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The standard Gibbs free energy change AG for a reaction at a given temperature ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The oxidation of SO 2 to SO 3 is one of the reactions involved in the formation of acid rain. If you want to predict the spontaneous direction of the reaction for a specific mixture of the gases, you...
-
(a) Using values in Appendix 2A, calculate the standard Gibbs free energy for the vaporization of water at 25.0C, 100.0C, and 150.0C. (b) What should the value at 100.0C be? (c) Why is there a...
-
Calculate the standard enthalpy, entropy, and Gibbs free energy for each of the following reactions at 298 K by using data in Appendix 2A. For each case, confirm that the value obtained from the...
-
1. Insurance Act, RSBC 1996 c226 Read Parts 1 and 2 of this statute and describe any changes to the standard commonlaw rules for contracts that you notice. 2. KP Pacific Holdings Ltd. and Churchland...
-
Reconsider the state-transition diagram in Figure. Describe, in words or with a diagram, a similar state-transition diagram for a system with three processes and a single resource type with two units...
-
A flower shop uses 800 clay pots a month. The pots are purchased at $2 each. Annual carrying costs are estimated at $0.60 per pot per year and ordering costs are $20 per order. The manager has been...
-
Describe normal flow, sub-flow, and alternate flow. How do they differ?
-
Pat Colt is auditing the financial statements of Manning Company. The following is a summary of the uncorrected misstatements that Colt has identified during the last three years. These misstatements...
-
Identify and discuss either the security features or return policy attached to the buying process on the website of THE BAY company. Please only speak to one of these. Is this clearly outlined during...
-
What is the molality of acetone, C 3 H 6 O, in an aqueous solution for which the mole fraction of acetone is 0.112?
-
Permanganate ions are powerful oxidizing agents used in water treatment facilities to remove metals, such as iron, and toxic and malodorous chemicals, such as H 2 S. If you are using permanganate...
-
What is the specificity using this cut-point? Genetics, Obstetrics Precise quantification of smoking during pregnancy is difficult in retrospective studies. Routinely collected blood specimens from...
-
(a) A parcel containing two components was received and the invoice discloses the following: Material I Material II Insurance 1,000 kgs @* 20 per kg 1,200 kgs @* 15 per kg 950 Sales tax Freight...
-
Consider the graph of y = f(x) below. 2 -1 0 -14 + 1 I +2 X (a) Find all values for which f(x) is discontinuous. (b) For each point in (a), describe where the 3-part definition fails. (c) Classify...
-
Enbridge assigns this project a cost of equity of 8% and a cost of debt of 5.48%. Treasury determines the optimal capital structure for the project is 75% debt and 25% equity. Using this information...
-
Cash Accounts Receivable Allowance for Doubtful Accts Inventory Prepaid Expenses Property, Plant, & Equipment Accounts Payable Accrued Liabilities Notes Payable Taxes Payable Long-term Debt Other...
-
A Find T(t) and N(t) for: r (+) = (+, sint- + cost, cost ++ sint) Part B) Find the Curvature
-
Discuss some problems that arise when pricing the transfer of intellectual property.
-
Feller Company purchased a site for a limestone quarry for $100,000 on January 2, 2019. It estimate that the quarry will yield 400,000 tons of limestone. It estimates that its retirement obligation...
-
Why does the energy of a rotating molecule depend on l , but not on m l ?
-
Are the real functions listed in Equations (18.62) and (18.63) eigenfunctions of l z ? Justify your answer.
-
Spatial quantization was discussed in Section 18.8. Suppose that we have a gas consisting of atoms, each of which has a nonzero angular momentum. Are all of their angular momentum vectors aligned?
-
Comprehensive Problem: Reviewing the Accounting Cycle Talbot Riding Stables provides stables, care for animals, and grounds for riding and showing horses. The account balances at the beginning of...
-
Required Information Use the following Information for the Exercises 8-10 below. (Algo) [The following information applies to the questions displayed below.] Hemming Company reported the following...
-
Expanding the discourse to encompass the transformative potential of central bank digital currencies (CBDCs) in reshaping the monetary landscape and redefining the role of traditional banking...

Study smarter with the SolutionInn App


