Question: (a) Using values in Appendix 2A, calculate the standard Gibbs free energy for the vaporization of water at 25.0C, 100.0C, and 150.0C. (b) What should
(a) Using values in Appendix 2A, calculate the standard Gibbs free energy for the vaporization of water at 25.0°C, 100.0°C, and 150.0°C.
(b) What should the value at 100.0°C be?
(c) Why is there a discrepancy?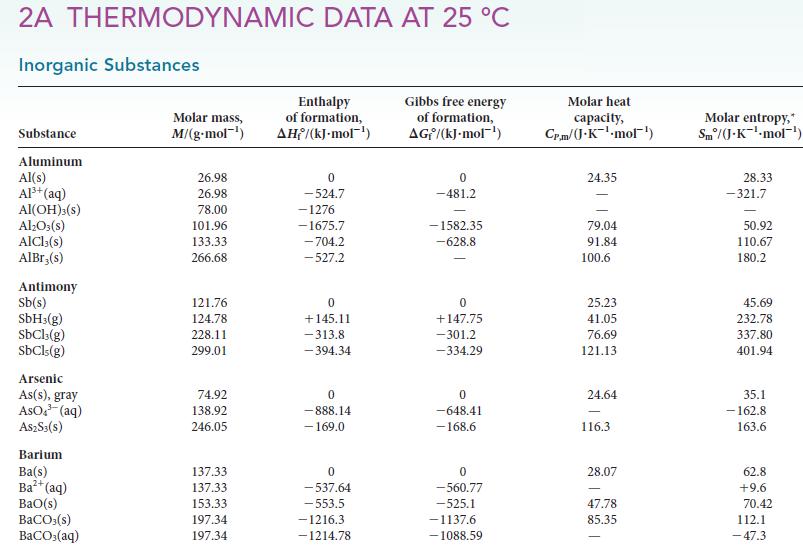
2A THERMODYNAMIC DATA AT 25 C Inorganic Substances Substance Aluminum Al(s) Al+ (aq) Al(OH)3(S) AlO3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray ASO (aq) A$2S3(S) Barium Ba(s) Ba+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K--mol) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy, Sm/(J.K-mol-) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
a 857 kJ at 298 K 035 kJ at 373 K 629 ... View full answer

Get step-by-step solutions from verified subject matter experts


