Calculate the unknown concentration of the ion in each of the following cells: 2+ (a) Pb(s) Pb+
Question:
Calculate the unknown concentration of the ion in each of the following cells: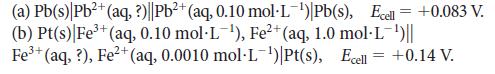
Transcribed Image Text:
2+ (a) Pb(s) Pb²+ (aq, ?)||Pb²+ (aq, 0.10 mol-L-¹) Pb(s), Ecell = +0.083 V. (b) Pt(s)| Fe³+ (aq, 0.10 mol-L-¹), Fe²+ (aq, 1.0 mol-L-¹)|| 2+ Fe³+ (aq, ?), Fe²+ (aq, 0.0010 mol-L-¹)|Pt(s), Ecell = +0.14 V.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a The cell notation can be written as Pbs Pb aq Pb aq 010 mol L Pbs Since the cell potential Ecell i...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The concentration of Fe3+ ion in a sample of H2O is 335.0 ppm. What mass of Fe3+ ion is present in 3,450 mL of H2O, which has a density of 1.00 g/mL?
-
Is the criterion 6 3CO 2 ) 2 (aq) is found to be 9.11. However, the contribution to the pH from the autoprotolysis of water was ignored. Repeat the calculation of the pH of this solution, taking into...
-
Calcium acetate, Ca(CH 3 CO 2 ) 2 (aq), is used to treat patients with a kidney disease that results in high levels of phosphate ions in the blood. The calcium binds to the phosphates so that they...
-
Digital Camera Shop Inc. uses the lower-of-cost-or-market basis for its inventory. The following data are available at December 31. Instructions What amount should be reported on Digital Camera...
-
Payback, even and uneven cash flows. You have the opportunity to expand your business by purchasing new equipment for $159,000. You expect to incur cash fixed costs of $96,000 per year to use this...
-
Boreki Enterprise has the following 10 items in inventory. Theodore Boreki asks you, a recent OM graduate, to divide these items into ABC classification. (a) Develop an ABC classification system for...
-
Diagnostic Services Inc. (DSI) is a new company. It has been in business for only one year, offering diagnostic services to physicians in the Tampa/St. Petersburg, Miami, and Orlando markets. DSI...
-
Following are the remarks of a prominent member of the U.S. Congress. Explain why you agree or disagree. The plain fact of the matter is that inflation accounting is a premature, imprecise, and...
-
Image transcription text QUESTION 1 Diffusion (a) The diffusion coefficients for carbon in nickel are given at two temperatures as shown in Table 1: Table 1 diffusion coefficients for carbon in...
-
Calculate the pH and pOH of (a) A solution that is 0.23 m Na 2 HPO 4 (aq) and 0.18 m Na 3 PO 4 (aq); (b) A solution that is 0.45 m Na 2 HPO 4 (aq) and 0.62 m Na 3 PO 4 (aq); (c) A solution that is...
-
Suppose that 1.773 g of impure barium hydroxide is dissolved in enough water to produce 200. mL of solution and that 25.0 mL of this solution is titrated to the stoichiometric point with 13.1 mL of...
-
Suppose that x* is a local solution of maxxG f(x). Then H+(x*) D(x*) = . Unfortunately, the set of feasible directions does not exhaust the set of relevant perturbations, and we need to consider a...
-
In 1969, the rock and roll band the Rolling Stones released a song entitled, "You Can't Always Get What You Want." In writing this song, lead singer Mick Jagger, who studied briefly at the London...
-
In early September Janet Huntley, the newly-appointed planner for MacDonald Refrigeration Ltd (MRL) of Sarnia, Ontario began working on MRLs production plan for the upcoming calendar year. The plan...
-
Complete the General Journal, Creditors ledger, and Debtors ledger using the transactions below. SHOW ALL WORKINGS, the format of the journal and ledgers are provided below: 1. 2. 3. 4. 5. 6. 7....
-
Estimation and Analysis of Demand for Brightness Detergents: Al Sharjah Detergents and Clearing Ind LLC based in Sharjah, UAE, is a leading producer and marketer of household laundry detergent and...
-
Use the data in housingdata.xls to answer the following questions. (a) Consider a simple linear regression model to explain the house price in terms of square footage: price = 0 + 1sqrf t + u...
-
Define the terms work in process (WIP) inventory, cost of goods manufactured (COGM), and cost of goods sold (COGS).
-
What is removed during each of the three stages of wastewater treatment: primary, secondary, and tertiary? During which state would you expect items to be recovered that were accidentally flushed,...
-
The racemization process described in the previous problem also occurs in acidic conditions. Draw a mechanism for the racemization process in aqueous acid.
-
Draw all four -hydroxyaldehydes that are formed when a mixture of acetaldehyde and pentanal is treated with aqueous sodium hydroxide.
-
Identify all of the different -hydroxyaldehydes that are formed when a mixture of benzaldehyde and hexanal is treated with aqueous sodium hydroxide.
-
Using The Porter Diamond Model, what are the Shanghai Port City Competitive Advantages?
-
Does the following series converge or diverge? 1 1 n 9 10 n n = 1 n
-
Identify weather the following series converge or diverge? Inn n 7n+3 n = 1

Study smarter with the SolutionInn App


