Question:
Calcium acetate, Ca(CH3CO2)2(aq), is used to treat patients with a kidney disease that results in high levels of phosphate ions in the blood. The calcium binds to the phosphates so that they can be excreted. If you are using calcium acetate for this purpose, it is important to know the pH of the solution to avoid complications in the treatment. Estimate the pH of 0.15 m Ca(CH3CO2)2(aq) at 25°C.
ANTICIPATE The CH3CO2– ion is the conjugate base of a weak acid; so the solution will be basic and you should expect pH > 7.
PLAN Use the procedure in Toolbox 6D.2, taking the initial concentration of base from the concentration of added salt. Calculate Kb for the basic anion from Ka for its conjugate acid. Convert pOH into pH by using Eq. 5b of Topic 6B (pH + pOH = 14.00).
What should you assume? As in Example 6D.2, you can make two assumptions initially: (1) because the protonation of the weak base is so small, the concentration of acetate ions retains its initial value; (2) the autoprotolysis of water does not affect the pH significantly. Be sure to verify these assumptions at the end of the calculation.
Example 6D.2
Although there are extensive tables available for the pKa of weak acids, you might be dealing with an unknown acid or a known acid at an unlisted temperature. You could then use a procedure like this to determine the Ka and pKa. The pH of a 0.010 m aqueous solution of a certain carboxylic acid is 2.95. What are its Ka and pKa?
ANTICIPATE All carboxylic acids are weak acids; therefore, expect Ka ≪ 1.
PLAN Calculate the hydronium ion concentration from the pH and then calculate the value of Ka from the equilibrium concentration of the acid and the hydronium ion concentration.
What should you assume? As in Example 6D.1, because pH
Example 6D.1
Acetic acid is a weak acid commonly found in both laboratory and household, but to what extent have its molecules actually been deprotonated? Calculate the pH and percentage deprotonation of CH3COOH molecules in 0.080 m CH3COOH(aq), given that Ka for acetic acid is 1.8 * 10–5.
ANTICIPATE Because the solution is that of an acid, expect pH
PLAN Following the procedure in Toolbox 6D.1, write the proton transfer equilibrium and construct the equilibrium table with concentrations in moles per liter.
What should you assume? You can make two assumptions, but they need to be verified at the end of the calculation.
(1) Deprotonation is so slight that the equilibrium concentration of the acid is approximately the same as its initial concentration.
(2) The autoprotolysis of water does not contribute significantly to the pH.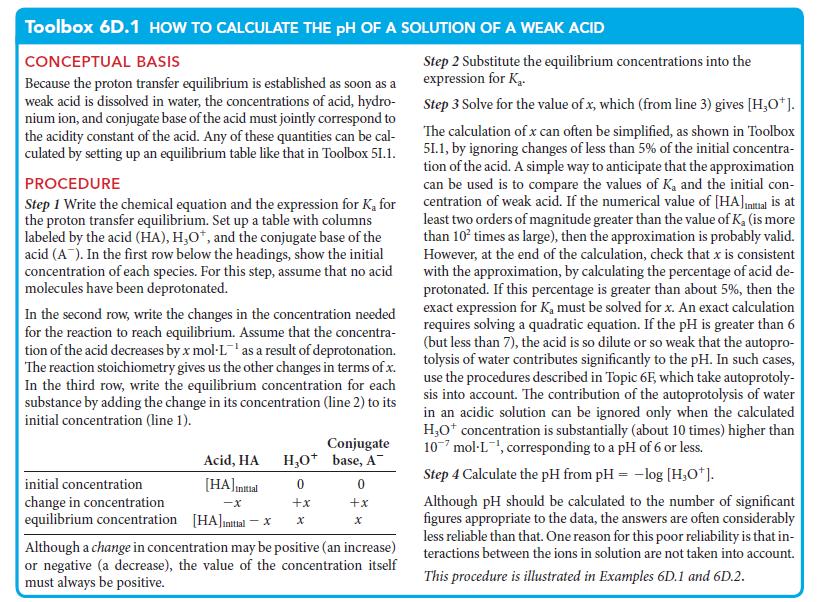
Transcribed Image Text:
Toolbox 6D.1 HOW TO CALCULATE THE PH OF A SOLUTION OF A WEAK ACID
CONCEPTUAL BASIS
Because the proton transfer equilibrium is established as soon as a
weak acid is dissolved in water, the concentrations of acid, hydro-
nium ion, and conjugate base of the acid must jointly correspond to
the acidity constant of the acid. Any of these quantities can be cal-
culated by setting up an equilibrium table like that in Toolbox 51.1.
PROCEDURE
Step 1 Write the chemical equation and the expression for K₂ for
the proton transfer equilibrium. Set up a table with columns
labeled by the acid (HA), H₂O*, and the conjugate base of the
acid (A). In the first row below the headings, show the initial
concentration of each species. For this step, assume that no acid
molecules have been deprotonated.
In the second row, write the changes in the concentration needed
for the reaction to reach equilibrium. Assume that the concentra-
tion of the acid decreases by x mol-L¹ as a result of deprotonation.
The reaction stoichiometry gives us the other changes in terms of x.
In the third row, write the equilibrium concentration for each
substance by adding the change in its concentration (line 2) to its
initial concentration (line 1).
initial concentration
change in concentration
equilibrium concentration
Acid, HA
[HA] initial
-X
[HA]initial - x
H₂O+
0
+x
X
Conjugate
base, A
0
+x
X
Although a change in concentration may be positive (an increase)
or negative (a decrease), the value of the concentration itself
must always be positive.
Step 2 Substitute the equilibrium concentrations into the
expression for K₂.
Step 3 Solve for the value of x, which (from line 3) gives [H₂O*].
The calculation of x can often be simplified, as shown in Toolbox
51.1, by ignoring changes of less than 5% of the initial concentra-
tion of the acid. A simple way to anticipate that the approximation
can be used is to compare the values of K, and the initial con-
centration of weak acid. If the numerical value of [HA]Initial is at
least two orders of magnitude greater than the value of K, (is more
than 10² times as large), then the approximation is probably valid.
However, at the end of the calculation, check that x is consistent
with the approximation, by calculating the percentage of acid de-
protonated. If this percentage is greater than about 5%, then the
exact expression for K, must be solved for x. An exact calculation
requires solving a quadratic equation. If the pH is greater than 6
(but less than 7), the acid is so dilute or so weak that the autopro-
tolysis of water contributes significantly to the pH. In such cases,
use the procedures described in Topic 6F, which take autoprotoly-
sis into account. The contribution of the autoprotolysis of water
in an acidic solution can be ignored only when the calculated
H₂O* concentration is substantially (about 10 times) higher than
10 mol-L¹, corresponding to a pH of 6 or less.
Step 4 Calculate the pH from pH = -log [H₂O*].
Although pH should be calculated to the number of significant
figures appropriate to the data, the answers are often considerably
less reliable than that. One reason for this poor reliability is that in-
teractions between the ions in solution are not taken into account.
This procedure is illustrated in Examples 6D.1 and 6D.2.







