Compare Figs. 14.38 and 14.40. Why are they different? Because B2 is known to be paramagnetic, the
Question:
Compare Figs. 14.38 and 14.40. Why are they different? Because B2 is known to be paramagnetic, the p2p and s2p MOs must be switched from our first prediction. What is the rationale for this? Why might one expect the s2p to be lower in energy than the p2p? Why can’t we use diatomic oxygen to help us decide whether the s2p or is lower in energy?
Fig. 14.38
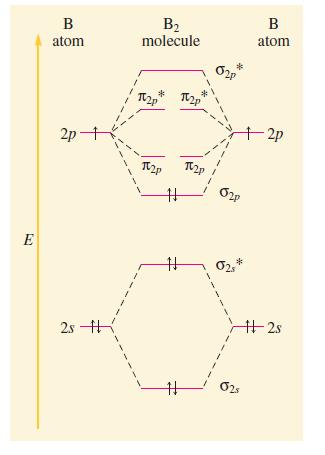
Fig. 14.40
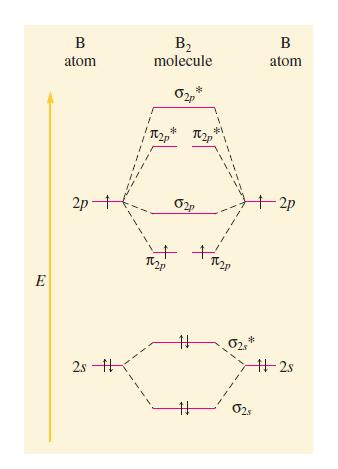
Transcribed Image Text:
E B atom 2p+ 2s B₂ molecule TV2P TUZP # # # #1 TV2P TU2p 1 I 1 020 B atom 025 -2p #2s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
Fig 1438 and 1440 differ because B2 is a paramagnetic molecule This means that the spin of the electrons is not paired up and the p2p and s2p MOs must ...View the full answer

Answered By

Hande Dereli
Enthusiastic tutor, skilled in ACT and SAT tutoring. Raised one student's score on the SATs from 1100 combined to 1400. Graduated with a 3.9 GPA from Davidson College and led a popular peer tutoring group for three years. Scored in the top 0.06% in the nation on the SATs. The real reason I'm the one to help you nail the test? Results. Clients invariably praise my ability to listen and communicate in a low-stress, fun way. I think it's that great interaction that lets me raise retest SAT scores an average of 300 points.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The life in hours of a battery is known to be approximately normally distributed, with standard deviation = 1.25 hours. A random sample of 10 batteries has a mean life of hours. (a) Is there evidence...
-
The frequency response of a stable LTI system is known to be real and even. Is the inverse system stable?
-
The half-life of a radioactive isotope is known to be exactly 1 h. (a) What fraction of a sample would be left after exactly 3 h: (1) one-third, (2) one-eighth, or (3) one-ninth? (b) What fraction of...
-
If OPEC raised the price of oil high enough, would that be sufficient to promote an efficient energy mix?
-
In a survey of men in the United States (ages 20 29), the mean height was 69.4 inches with a standard deviation of 2.9 inches. (a) What height represents the 90th percentile? (b) What height...
-
Fill in the blanks in each of the following statements: a) The logical unit that receives information from outside the computer for use by the computer is the _________. b) The process of instructing...
-
Can you present a graphic that presents the payroll disbursement amounts by date for the contact employee who has been terminated but has been paid after termination (i.e., ghost employees)?
-
I know headquarters wants us to add that new product line, said Dell Havasi, manager of Billings Companys Office Products Division. But I want to see the numbers before I make any move. Our divisions...
-
1-2. Draw graphs of the following functions using transformations: 1. y = 2x-1 x-2' 2. y log2x+1|- 3.
-
Childrens Best Hope (CBH) provides day care ser-vices to low income families. CBH bills the state for its services under a service contract. Billings for the first four months of 2013 are anticipated...
-
Ronald Robinson, Wyman Robinson, and Friendly Discount Auto Sales (appellants) appeal from the granting of summary judgment in favor of appellee Mike Durham (Durham). The facts material to this...
-
Explain the difference between the and MOs for homonuclear diatomic molecules. How are bonding orbitals and antibonding orbitals different? Why are there two MOs and one's MO? Why is the p MOs...
-
Distinguish between a diffusion-controlled reaction and an activationcontrolled reaction. Do both have activation energies?
-
Traditional Bidayuh people use bamboo rafts to navigate down the smaller rivers of their heartland in the Padawan region. The standard size of bamboo rafts has a dimension of 1 1 0 cm by 2 0 5 cm by...
-
Is the Balance Sheet a flow or a static statement?
-
Palmetto Tree Service is a leading seller of trees to homeowners in the Southwest US . The company sells the trees for $ 3 0 0 each, which includes installation. Davis Griffin, the company's owner,...
-
Would the sales manager's salary ( included in marketing, distribution, and customer - service costs ) be accounted for any differently if the Corporation were a merchandising - sector company...
-
Select a coder/biller for a health care provider or facility andconduct an interview to review the process the coder/biller uses tosatisfy reimbursement requirements for billing purposes. Writepaper...
-
The data in Travel show the average traffic on Google recorded at the beginning of each month from January 2004 to August 2010 for searches from the United States concerning travel (scaled to the...
-
Find the equations of the ellipses satisfying the given conditions. The center of each is at the origin. Passes through (2, 2) and (1, 4)
-
Dissociation of a diatomic molecule, X 2 (g) 2 X(g)occurs at 500 K. The equilibrium state of the reaction is shown in 1 and the equilibrium state in the same container after a change has occurred is...
-
A sample of ammonium carbamate, NH 4 (NH 2 CO 2 ), of mass 25.0 g was placed in an evacuated flask of volume 0.250 L and kept at 25C. At equilibrium, 17.4 mg of CO 2 was present. What is the value of...
-
Interpret the following verse from Coleridges Rime of the Ancient Mariner: Water, water, every where, And all the boards did shrink; Water, water every where, Nor any drop to drink.
-
3. For the finale of her act, famed balloonist Mer- cedes Corominas would suspend herself from a trapeze 10 m below the basket, and she would kick a ball with lit sparklers towards a confeder- ate on...
-
Electric field lines are diverging from each other in a region of space, as shown in the diagram at right. Four adjacent field lines intersect the corners of a square with area 2 cm, the lines being...
-
3. Imagine you are canoeing across a lake at night with a friend. You have a flashlight in your canoe so you can see where you are going. Now, for some unknown reason, your so-called friend tips the...

Study smarter with the SolutionInn App


