Consider 2.00 mol of a monatomic ideal gas that is taken from state A (P A
Question:
Consider 2.00 mol of a monatomic ideal gas that is taken from state A (PA × 2.00 atm, VA = 10.0 L) to state B (PB = 1.00 atm, VB = 30.0 L) by two different pathways:
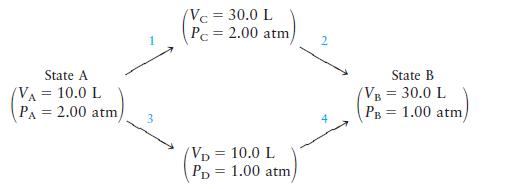
Calculate q, w, ΔE, and ΔH for both pathways.
Transcribed Image Text:
State A (VA = 10.0 L PA= 2.00 atm/ (Vc = 30.0 L Pc = 2.00 atm, VD = 10.0 L PD 1.00 atm/ = State B (VB = 30.0 L PB 1.00 atm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Before we do any calculations it is useful to summarize the processes described above by using the PV diagram shown in Fig 95 Step 1 Notice from Fig 9...View the full answer

Answered By

Moses mwangi
With prior writing experience, be sure that I will give a great grade, If not an A+, it will be something close to this. My reviews speaks it all, Try me!!
4.80+
78+ Reviews
157+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider a sample containing 5.00 moles of a mona-tomic ideal gas that is taken from state A to state B by the following two pathways: For each step, assume that the external pressure is constant and...
-
Consider 2.00 moles of an ideal gas that is taken from state A (PA = 2.00 atm, VA = 10.0 L) to state B (PB = 1.00 atm, VB = 30.0 L) by two different pathways. These pathways are summarized in the...
-
Consider a sample containing 2.00 moles of a monatomic ideal gas that undergoes the following changes: For each step, assume that the external pressure is constant and equals the final pressure of...
-
Does the fact that the strategic petroleum reserve has never been used to offset shortfalls caused by an embargo mean that the money spent in creating the reserve has been wasted? Why or why not?
-
Derive the expression for the phase paths of the plane pendulum if the total energy is E > 2mgl. Note that this is just the case of a particle moving in a periodic potential U () = mgl (1 - cos ).
-
Write an UPDATE statement to change the phone number of employee with EmployeeNumber 11 to 360-287-8810. Run this SQL statement.
-
Nereus Montemayor was an employee of VZ Hogs, a company that raises hogs and produces hog feed. VZ Hogs used an extruder manufactured by Sebright Products, Inc. to create hog feed out of discarded...
-
Cost allocation to divisions. Forber Bakery makes baked goods for grocery stores, and has three divisions: Bread, Cake, and Doughnuts. Each division is run and evaluated separately, but the main...
-
Calculate Inventory Conversion Period, Receivables Conversion Period, the Payment Conversion Period, and the Operating Cycle for Innovation Inc. given the following information. Use 365 to get an...
-
When 1.00 L of 1.00 M Ba(NO 3 ) 2 at 25.0C is mixed with 1.00 L of 1.00 M Na 2 SO 4 at 25C in a calorimeter, the white solid BaSO 4 forms, and the temperature of the mixture increases to 28.1C....
-
A balloon is inflated to its full extent by heating the air inside it. In the final stages of this process the volume of the balloon changes from 4.00 10 6 L to 4.50 10 6 L by addition of 1.3 10 8...
-
B-Flat Music Supplies Ltd. earned dividends of \(\$ 30,000\), interest of \(\$ 12,000\), adjusted rent of \(\$ 50,000\), and gross income from its primary business of \(\$ 63,000\) in one taxable...
-
IM&C's guano project. Revised analysis with immediate expensing of investment expenditures. Period 0 1 2 3 4 5 6 7 Panel A Capital Investment 1. Investment in fixed assets -12,000 2. Sale of fixed...
-
How can Mary Kay encourage IBCs to embrace new technology? How can Mary Kay balance the use of mainstream influencers with their IBCs as influencers without losing IBCs trust?
-
How has Covid-19 impacted (a) the beauty industry, in general, and (b) specifically, Mary Kay, in terms of sales methods and technology?
-
What need to be learned and applied from the past experiences to the new phenomena of pure drinking water in Egyptian Villages?
-
What is e-commerce? How has e-commerce evolved over the years? What are the benefits and challenges of e-commerce? What are the latest trends in e-commerce, both from a business and technological...
-
What is continuous flow processing? Give at least three examples of products that might use continuous flow processing.
-
A 20-cm-square vertical plate is heated to a temperature of 30oC and submerged in glycerin at 10oC. Calculate the heat lost from both sides of the plate.
-
(a) Write the valence electron configurations of the alkali metal atoms. (b) Explain why the alkali metals are strong reducing agents in terms of electron configurations, ionization energies, and the...
-
Identify the oxidation number of germanium in the following compounds and ions: (a) GeO 4 4 ; (b) K 4 Ge 4 Te 10 ; (c) Ca 3 GeO 5 .
-
Complete and balance the following equations: (a) BO3(s) + Mg(1) (b) Al(s) + Cl(g) (c) Al(s) + O(g)
-
what types of dangers does The Haunted Witch Trials have a duty to warn its patrons?
-
Describe a community service research report elaborating its purpose , history , employees, voluntarees, responsibipties clients reporg etc.
-
How does quality as a measure of reliability create competitive advantage for a firm? It eliminates the need for customer service. It improves responsiveness to customer needs. It increases value for...

Study smarter with the SolutionInn App


