Question: Consider Fig. 17.8. Suppose that instead of having a nonvolatile solute in the solvent in one beaker, the two beakers have different volatile liquids. That
Consider Fig. 17.8. Suppose that instead of having a nonvolatile solute in the solvent in one beaker, the two beakers have different volatile liquids. That is, suppose one beaker contains liquid A (Pvap 5 50 torr) and the other beaker contains liquid B (Pvap 5 100 torr). Explain what happens as time passes. How is this similar to the first case shown in the figure? How is it different?
Fig. 17.8
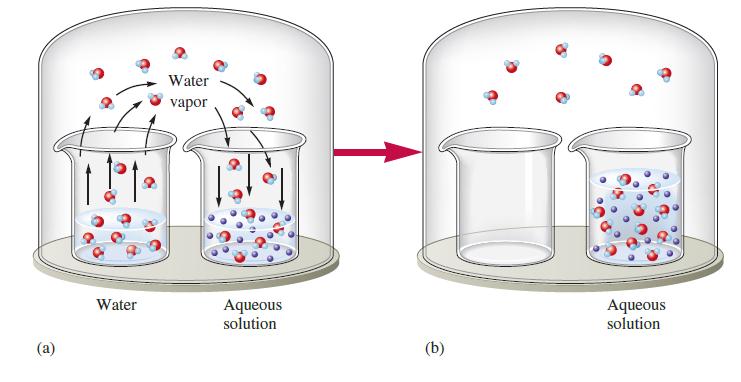
(a) Water Water vapor Aqueous solution (b) Aqueous solution
Step by Step Solution
3.42 Rating (168 Votes )
There are 3 Steps involved in it
In Fig 178 if the two beakers contain different volatile liquids instead of a nonvolatile solute in ... View full answer

Get step-by-step solutions from verified subject matter experts


