Consider only the species (at standard conditions) [mathrm{Na}^{+}, mathrm{Cl}^{-}, mathrm{Ag}^{+}, mathrm{Ag}, mathrm{Zn}^{2+}, mathrm{Zn} text {, and }
Question:
Consider only the species (at standard conditions)
\[\mathrm{Na}^{+}, \mathrm{Cl}^{-}, \mathrm{Ag}^{+}, \mathrm{Ag}, \mathrm{Zn}^{2+}, \mathrm{Zn} \text {, and } \mathrm{Pb}\]
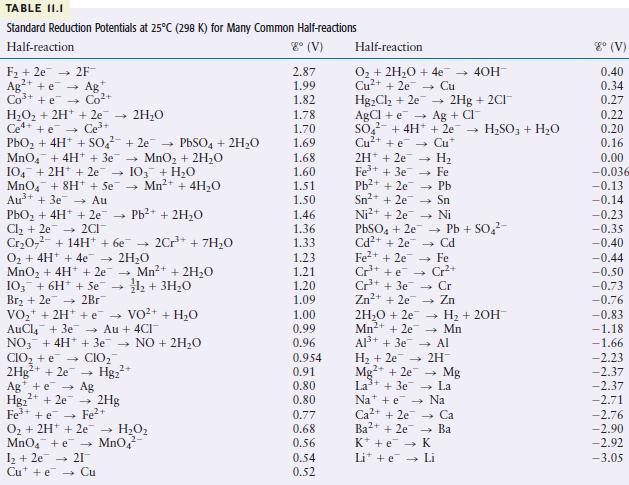
in answering the following questions. Give reasons for your answers. (Use data from Table 11.1.)
a. Which is the strongest oxidizing agent?
b. Which is the strongest reducing agent?
c. Which species can be oxidized by \(\mathrm{SO}_{4}{ }^{2-}(a q)\) in acid?
d. Which species can be reduced by \(\mathrm{Al}(s)\) ?
Transcribed Image Text:
TABLE II.I Standard Reduction Potentials at 25C (298 K) for Many Common Half-reactions Half-reaction 8 (V) F +2e 2F Ag+ e Ag Co+ + e Co+ HO + 2H+ + 2e 2HO Ce4+ +e Ce+ PbO + 4H+ + SO +2e PbSO4 + 2HO MnO4 + 4H+ + 3e MnO + 2HO 104 + 2H+ + 2e 103 + HO MnO4 + 8H+ + Se Mn+ + 4HO Au+ + 3e Au PbO + 4H+ + 2e Pb+ + 2HO Cl +2e2CI CrO + 14H+ + 6e 2Cr+ + 7H0 O + 4H+ + 4e 2HO MnO + 4H+ + 2e 4 Mn+ + 2HO 1+ 3HO 103 + 6H + Se Br +2e 2Br VO + 2H+ + VO+ AuCl4 + 3e Au + 4CI NO3 + 4H+ + 3e NO + 2HO CIO + e CIO 2Hg+ + 2e Hg+ Ag + e 2+ Hg+ + 2e Fe+ + e O + 2H+ 2e MnO4 + e 1 +2e 21 Cute Cu Ag 2Hg Fe+ HO MnO4 + HO 2.87 1.99 1.82 1.78 1.70 1.69 1.68 1.60 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.99 0.96 0.954 0.91 0.80 0.80 0.77 0.68 0.56 0.54 0.52 Half-reaction O + 2HO + 4e Cu+ + 2e Cu HgCl +2e AgCl + e SO + 4H+ Cu+ + e 2H+2e7 4 H 1 Fe+ + 3e7 Pb+ + 2e7 Fe Pb - Sn Ni Sn+ + 2e Ni+ + 2e PbSO4 + 2e7 Cd+ + 2e Fe+ + 2e Cr+ + e Cr+ + 3e Zn+ + 2e7 2HO +2e Mn+ + 2e Al+ + 3e 2Hg + 2CI+ Ag + CI + 2e- HSO3 + HO Cu* Cd - Fe Cr+ Cr Zn - H + 2OH- Mn Al Pb + SO - 40H- H + 2e 2H Mg2+ + 2e La+ + 3e7 Nae Ca+ + 2e7 Ba2+ + 2e K + e Li + e Mg La Na Ca Ba K Li 8 (V) 0.40 0.34 0.27 0.22 0.20 0.16 0.00 -0.036 -0.13 -0.14 -0.23 -0.35 -0.40 -0.44 -0.50 -0.73 -0.76 -0.83 -1.18 -1.66 -2.23 -2.37 -2.37 -2.71 -2.76 -2.90 -2.92 -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To answer these questions we will refer to the reduction potentials listed in the provided table Standard reduction potentials indicate the tendency o...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the relation shown in Figure 30.2(d). How would it appear to a user with classification U? Suppose that a classification U user tries to update the salary of Smith to $50,000; what would be...
-
On October 10, the stockholders' equity section of Sherman Systems appears as follows. Common stock-$10 par value, 5,050 shares authorized, issued, and outstanding Paid-in capital in excess of par...
-
Consider only the species (at standard conditions) Br 2 , Br 2 , H + , H 2 , La 3+ , Ca, Cd in answering the following questions. Give reasons for your answers. a. Which is the strongest oxidizing...
-
Suppose there are two identical forest plots except that one will be harvested and left as is while the second will be cleared after the harvest and turned into a housing development. In terms of...
-
A gun fires a projectile of mass 10kg of the type to which the curves of Figure 2-3 apply. The muzzle velocity is 140m/s. Through what angle must the barrel be elevated to hit a target on the same...
-
Figure 3.24 shows a closed container holding water and oil. Air at 34 kPa below atmospheric pressure is above the oil. Calculate the pressure at the bottom of the container in kPa(gage). 0,25 m Air...
-
What are the two categories of data mining and knowledge discovery software?
-
Grand Prix Displays Inc. manufactures and assembles automobile instrument panels for both Yokohama Motors and Detroit Motors. The process consists of a just-in-time product cell for each customers...
-
Who developed the relational model, when, and why?
-
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following oxidations (under standard conditions in acidic solution). a. oxidizes...
-
The saturated calomel electrode, abbreviated SCE, is often used as a reference electrode in making electrochemical measurements. The SCE is composed of mercury in contact with a saturated solution of...
-
A friend calculates a variance and reports that it is 25.0. How do you know that he has made a serious calculation error?
-
Why are taxes necessary? Taxation is essential to the functioning of the modern state. To pay for the things that we need to live our daily lives, such as water, roads, security, and education, the...
-
Consider a list of 8 real numbers: 20, 40, 10, 50, 80, 40, 60, 30. Compute the Fourier coefficients Fo and F, using the DFT. Repeat (a) using the FFT. Verify that you get the same results as above....
-
On January 1, 2023, Vin Diesel Inc purchases a fleet of six vehicles for $50,000 each. They pay cash for the purchase. Vehicles have zero salvage value for depreciation calculations. Vin Diesel...
-
Yates Control System (YCS): Will the Bank Make the Loan? (available in Course-Pack). Taking the role of the decision-maker, briefly discuss the following: YCS's past financial performance. What...
-
The balance sheet of Perez Printing shows $ 6 8 0 in inventory, $ 2 , 1 4 0 in fixed assets, $ 2 1 0 in accounts receivables, $ 2 5 0 in accounts payable, and $ 8 0 in cash. How much net working...
-
Owl-Eye Radiologists (OR) does various types of diagnostic imaging. Radiologists perform tests using sophisticated equipment. ORs management wants to compute the costs of performing tests for two...
-
Use Stokes' Theorem to evaluate f(y+sin x) dx+(z+cos y) dy+rdz, where C is the rve r(t) = (sint, cost, sin 2t), t = [0, 2].
-
Calculate the pH of 6.55 * 10 7 m HClO 4 (aq).
-
One of the largest uses of electricity is in the production of aluminum by electrolysis of its oxide dissolved in molten cryolite (Na 3 AlF 6 ). As an engineer, you might need to predict how much...
-
You are working in an analytical laboratory and have been asked to use the permanganate solution you prepared in Example 6K.1 to determine the concentration of bromide ions in a sample of groundwater...
-
Humans shed about 10M dead skin cells a day. These skin cells (or squames) are disc shaped and have density of water. As these skin cells can carry bacteria, it is important to know more about their...
-
Problem #5 (15 points) Provide a Matlab code utilizing "for" loop to compute the square power, square root, and sin(x) function of all integers between 9 and 11. Problem # 6 (15 points) At the...
-
Determine how many kilograms of steel will be needed to make 10,000 jumbo paperclips. The additional information you have is density of steel is 8050 kg/m 3 . The only tools that you can use are a...

Study smarter with the SolutionInn App


