Question: Consider the dissolution of CaCl 2 : CaCl(s) Ca+ (aq) + 2Cl(aq) AH = -81.5 kJ
Consider the dissolution of CaCl2: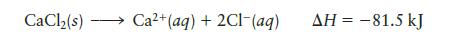
CaCl(s) Ca+ (aq) + 2Cl(aq) AH = -81.5 kJ
Step by Step Solution
3.50 Rating (153 Votes )
There are 3 Steps involved in it
The dissolution of CaCl2 is an exothermic process as indicated by ... View full answer

Get step-by-step solutions from verified subject matter experts


