Consider the following galvanic cell: A 15.0-mol sample of (mathrm{NH}_{3}) is added to the Ag compartment (assume
Question:
Consider the following galvanic cell:
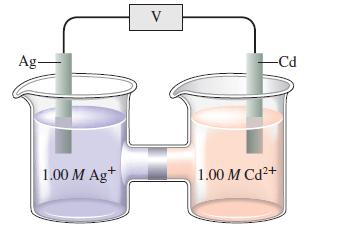
A 15.0-mol sample of \(\mathrm{NH}_{3}\) is added to the Ag compartment (assume 1.00 L of total solution after the addition). The silver ion reacts with ammonia to form complex ions as shown:
\(\mathrm{Ag}^{+}(a q)+\mathrm{NH}_{3}(a q) ightleftharpoons \mathrm{AgNH}_{3}{ }^{+}(a q)\)
\[K_{1}=2.1 \times 10^{3}\]
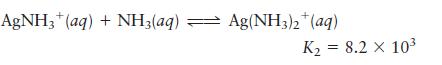
Calculate the cell potential after the addition of \(15.0 \mathrm{~mol}\) \(\mathrm{NH}_{3}\).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





