Describe completely the galvanic cell based on the following half-reactions under standard conditions: Ag + e Fe+
Question:
Describe completely the galvanic cell based on the following half-reactions under standard conditions:
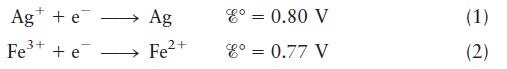
Transcribed Image Text:
Ag + e Fe+ + e Ag Fe2+ 8 = 0.80 V 8 = 0.77 V (1) (2)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Since a positive cell value is required reaction 2 must run in 8 cathode 080 V 8 anode 077 ...View the full answer

Answered By

Vincent Omondi
I am an extremely self-motivated person who firmly believes in his abilities. With high sensitivity to task and operating parameters, deadlines and keen on instructions, I deliver the best quality work for my clients. I handle tasks ranging from assignments to projects.
4.90+
109+ Reviews
314+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the standard galvanic cell based on the following half reactions Cu2+ + 2e- Cu Ag+ + e- Ag The electrodes in this cell are Ag(s) and Cu(s). Does the cell potential increase, decrease, or...
-
(A) Consider a galvanic cell based on the following half-reactions. Assuming the cell operates under standard conditions at 25 C, what is the spontaneous cell reaction? Under what nonstandard...
-
Consider the galvanic cell based on the following halfreactions: b. Calculate ÎGo and K for the cell reaction at 25oC. c. Calculate cell at 25oC when [Zn2+] = 0.10 M and [Fe2+] = 1.0 Ã...
-
Write a StockAccount client that builds an array of StockAccount objects, computes the total value of each account, and prints a report for the accounts with the largest and smallest values. Assume...
-
A particle of mass m has speed v = a/x, where x is its displacement. Find the force F(x) responsible.
-
In real-time interactive audio/video, what will happen if a packet arrives at the receiver site after the scheduled playback time?
-
Identify and describe the key attributes that might mitigate a red flag, suggesting that no bad act or compliance issues exist.
-
In the new system under development for Holiday Travel Vehicles, seven tables will be implemented in the new relational database. These tables are New Vehicle, Trade-in Vehicle, Sales Invoice,...
-
Ethel has an existing loan which she wishes to refinance to obtain a lower rate. Her home has appraised for 352,000. She has requested a new loan in the amount of 264,000 to pay off her old loan and...
-
Using the data in Table 11.1, calculate G for the reaction Is this reaction spontaneous? Cu+ (aq) + Fe(s) Cu(s) + Fe+ (aq)
-
Consider the following galvanic cell: A 15.0-mol sample of \(\mathrm{NH}_{3}\) is added to the Ag compartment (assume 1.00 L of total solution after the addition). The silver ion reacts with ammonia...
-
Solve the following equations for x. Give your answers to two decimal places. (a) 5x = 8 (b) 10 x = 50 (c) 1.2 x = 3 (d) 1000 1.05 x = 1500
-
Explain "who" pays the cost of government regulation of business. Be very specific in your answer. Distinguish between compliance costs and non-compliance costs. What happens when the costs cannot be...
-
Hilton partners with Lysol and the Mayo Clinic as it repositions its strategy around the core competencies of "clean and safe." Other hotels are similarly redesigning strategies and services to meet...
-
What is your opinion on how to strike a balance between investing in long-term resilience and tackling urgent vulnerabilities while prioritizing mitigation measures
-
Per the PCAOB, how long does the audit firms have to retain the audit papers/records? 17. What is the "rest period" of the firm if a member of the audit engagement team becomes CEO or CFO (or leading...
-
How do you calculate the annual share based compensation expenses for multiple years? And what specifically does share based compensation represent? How can I find how many options were granted and...
-
Some people claim that the scattergraph and the regression methods go hand in hand. Why?
-
QUESTION 2 The CEO of Farisha Hijab Sdn Bhd insisted on further investigation to be carried out that he also required Mr Muaz to conduct the analysis of variance for the material and labour of the...
-
Citric acid, which is extracted from citrus fruits and pineapples, undergoes three successive deprotonations with pK a values of 3.14, 5.95, and 6.39. Estimate the pH of (a) A 0.15 m aqueous solution...
-
Is the criterion 6 3CO 2 ) 2 (aq) is found to be 9.11. However, the contribution to the pH from the autoprotolysis of water was ignored. Repeat the calculation of the pH of this solution, taking into...
-
Is the criterion 6 In Example 6D.4, the pH of 0.15 m NH 4 Cl(aq) is found to be 5.04. However, the contribution to the pH from the autoprotolysis of water was ignored. Repeat the calculation of the...
-
(b) Rasool wants to rent an apartment in Cheras, Selangor next year. He surveyed a random sample of 60 apartments advertised on Senang.MY website. The monthly rent of the apartments follows a normal...
-
Provide a solution using MATLAB to verify the formula of a centroid for the given example problem. Formula is provided. explain the use of how you used MATLAB to verify the formula of the attached...
-
2. For each of the 2-periodic functions below, find the corresponding Fourier series. Unless stated otherwise, assume that each of the function g is defined on (,). (c) g7(t) = cos(t)| (d) gs(t) =...

Study smarter with the SolutionInn App


