Consider the reaction 2 NO 2 (g) 2 NO(g) + O 2 (g). If the initial
Question:
Consider the reaction 2 NO2(g) ⇌ 2 NO(g) + O2(g). If the initial molar concentration of NO2(g) is 0.030 mol · L–1, and c is the equilibrium molar concentration of O2(g) in moles per liter, which of the following expressions is the correct equilibrium relation?![]()
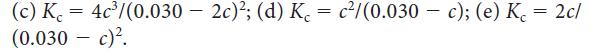
Transcribed Image Text:
(a) K c³; (b) Kc 2c²/(0.030 2c)²;
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Rishabh Ojha
During my undergraduate i used to participate as TA (Teaching Assistant) in several electronics and computers subject. I'm passionate about learning Computer Science as my bachelors are in Electronics but i learnt most of the Computer Science subjects on my own which Machine Learning also. At Present, i'm a working professional pursuing my career as a Machine Learning Engineer and i want to help others learn during my free hours, that's all the motivation behind giving tuition. To be frank i have no prior experience of tutoring but i have solved problems on opensource platforms like StackOverflow and github. ~Thanks
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The reaction 2 NO2 2 NO + O2 has the rate constant. k = 0.63 M-1s-1. Based on the units for k, is the reaction first or second order in NO2? If the initial concentration of NO2 is 0.100M, how would...
-
Consider the reaction 2 CO2 2 CO + O2 obtained after heating 1 kmol CO2 to 3000 K. Find the equilibrium constant from the shift in Gibbs function and verify its value with the entry in Table A.11....
-
What is the molarity of a solution containing 25.5 g KBr dissolved in enough water to make 1.75 L of solution? SORT You are given the mass of KBr and the volume of a solution and asked to find its...
-
The functions in Exercises 1128 are all one-to-one. For each function, a. Find an equation for f -1 (x), the inverse function. b. Verify that your equation is correct by showing that f( f -1 (x)) = x...
-
Capacitors C1 and C2 are connected in parallel by a resistor and two switches as shown in Figure. Capacitor C1 is initially charged to a voltage V0, and capacitor C2 is uncharged. The switches S are...
-
Newton Manufacturing produces scientific calculators. The models are N350, N450, and the N900. Newton has planned its distribution of these products around eight customer zones: Brazil, China,...
-
Consider a randomized block design with $k$ treatments and $b$ blocks.a. Derive the least squares estimators of the treatment effects using i) dummy coding and ii) deviation coding. b. Verify the...
-
The PDC Company was described earlier in this chapter. Refer to the PDC Companys projected monthly operating schedules in Table. PDCs sales are projected to be $80,000 in September 2011. A. Prepare...
-
Many colleges and universities are supported by the government, while others are supported by private organizations. What bearing does the source of support have on the determination of authoritative...
-
The following transactions occurred in November 202X for J. Kingslys Technical Staffing Agency: The chart of accounts for J. Kingsly Technical Staffing Agency is as follows: Your task is to do the...
-
In an experiment, 0.020 mol NO 2 was introduced into a flask of volume 1.00 L and the reaction 2 NO 2 (g) N 2 O 4 (g) was allowed to come to equilibrium at 298 K. (a) Using information in Table...
-
(a) Calculate the standard Gibbs free energies of formation of the halogen atoms X (g) at 1000. K from data available in Table 5G.2. (b) Show how these data correlate with the XX bond strength by...
-
1. If an ore isn't radioactive, then it isn't pitchblende. 2. All but the rats left the sinking ship. 3. A pesticide is dangerous if it contains DDT. 4. John Grisham writes only novels about lawyers....
-
Using real-world examples, discuss the consequences of an appreciation of a country's currency on the current account balance, inflation and economic growth.
-
Using real-world examples, evaluate the effectiveness of supply-side policies in reducing unemployment.
-
Using real-world examples, evaluate the effects of a tariff, or an international subsidy, or a quota on a specific market for the different stakeholders in an economy.
-
Express each complex number in its polar form. \(\sqrt{3}+j\)
-
Solve the IVP. Do not use Laplace transformation. \(\ddot{y}-y=t^{2}-\cos t, \quad y(0)=\frac{1}{2}, \dot{y}(0)=0\)
-
Paula purchases a 40% interest in Dancer Enterprises for $52,000 on January 2 of the current year. Dancer is organized as a partnership and has an income of $50,000 in the current year. Dancer also...
-
Find the reduced echelon form of each of the matrices given in Problems 120. c 1 26 + 4
-
Consider the following [4+4] cycloaddition process. Would you expect this process to occur through a thermal or photochemical pathway? Justify your answer with MO theory. +
-
Predict the major product(s) for each of the following thermal electrocyclic reactions: (a) (b) (c) Heat Heat
-
Predict the product for each of the following reactions: (a) (b) (c) Light Light
-
Let D= {(x, y): x + y < 1} C R and consider the Neumann BVP Au=0, (x,y) E D h, x + y = 1, where - Vu n denotes the normal derivative of u on OD, n is the unit normal vector to OD, and where h is a...
-
How do emergent structures, characterized by self-organizing principles and distributed decision-making authority, challenge traditional hierarchical models of organizational governance and redefine...
-
Xavier and Yolanda have original investments of $51,200 and $103,600, respectively, in a partnership. The articles of partnership include the following provisions regarding the division of net...

Study smarter with the SolutionInn App


