(a) Calculate the standard Gibbs free energies of formation of the halogen atoms X (g) at 1000....
Question:
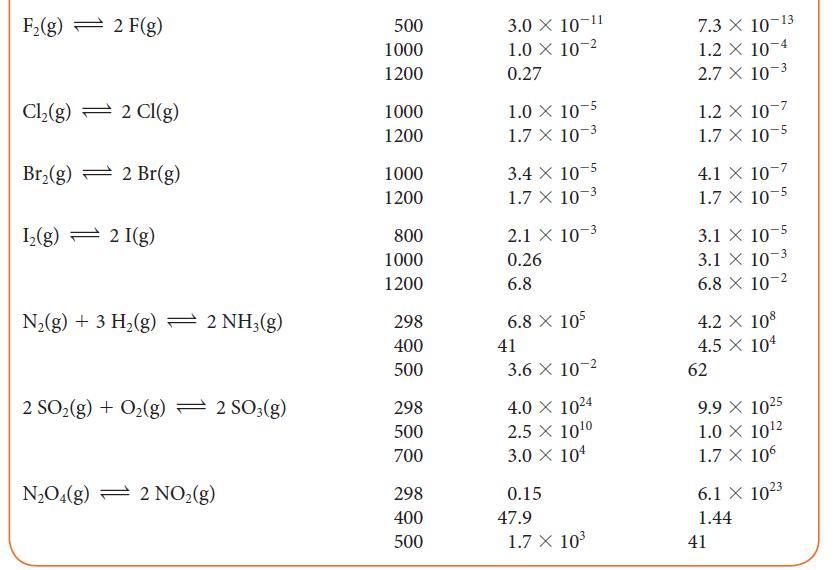
(a) Calculate the standard Gibbs free energies of formation of the halogen atoms X(g) at 1000. K from data available in Table 5G.2.
(b) Show how these data correlate with the X—X bond strength by plotting the standard Gibbs free energy of formation of the atoms against the bond dissociation energy and atomic number. Rationalize any trends you observe.
Transcribed Image Text:
TABLE 5G.2 Equilibrium Constants for Various Reactions T/K* Reaction H₂(g) + Cl₂(g) 2 HCl(g) H₂(g) + Br₂(g) 2 Hbr(g) H₂(g) + 1₂(g) = 2 HI(g) 2 BrCl(g) Br₂(g) + Cl₂(g) 2 HD(g) H₂(g) + D₂(g) 300 500 1000 300 500 1000 298 500 700 300 500 1000 100 500 1000 K 4.0 × 10³1 4.0 × 10¹8 5.1 X 108 1.9 X 10¹7 1.3 X 10¹⁰ 3.8 X 104 794 160 54 377 32 5 0.52 0.28 0.26 K 4.0 × 10³1 4.0 × 10¹8 5.1 X 108 1.9 X 10¹7 1.3 × 10¹0 3.8 X 104 794 160 54 377 32 5 0.52 0.28 0.26
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Halogen Fluorine Chlorine Bromine Iodine Bond Dissociation ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
In Exercises 1126, determine whether each equation defines y as a function of x. x + y = 16
-
Two batteries with emf E1 and E2 and internal resistances r1 and r2 are connected in parallel. Prove that if a resistor is connected in parallel with this combination, the optimal load resistance...
-
Noonan worked for Staples, the office supply store company. It has a Code of Ethics for employees. One provision states: "We expect you to keep accurate records and reports.. We do not permit.false...
-
Consider the fisher. barley data frame from Example 5.3. a. Use $\mathrm{R}$ to compute $S S_{E}$. What are the degrees of freedom for $S S_{E}$ ? b. A statistician analyzing fisher.barley forgot to...
-
Washington Co. and Vermont Co. have no domestic business. They have a similar dollar equivalent amount of international exporting business. Washington Co. exports all of its products to Canada....
-
The directors of a company require that all investment projects should be evaluated using either payback period or return on capital employed ( accounting rate of return ) . The target payback period...
-
Santana Rey created Business Solutions on October 1, 2020. The company has been successful, and its list of customers has grown. To accommodate the growth, the accounting system is modified to set up...
-
Consider the reaction 2 NO 2 (g) 2 NO(g) + O 2 (g). If the initial molar concentration of NO 2 (g) is 0.030 mol L 1 , and c is the equilibrium molar concentration of O 2 (g) in moles per liter,...
-
In an experiment, 0.100 mol SO 3 was introduced into a flask of volume 2.00 L and the reaction 2 SO 2 (g) + O 2 (g) 2 SO 3 (g) was allowed to come to equilibrium at 700 K. (a) Using information in...
-
When should a consolidated entity recognize a goodwill impairment loss? a. When the fair value of a reporting unit exceeds its respective carrying amount b. Whenever the entitys fair value declines...
-
Using real-world examples, discuss the merits of free trade versus protectionism.
-
Write the expression in the form \(D \sin (\omega t+\phi)\). \(3 \sin \omega t-\cos \omega t\)
-
Using real-world examples, evaluate the view that a government's fiscal policy can be both a cause of inequality and a means of reducing inequality.
-
Using real-world examples, evaluate the effectiveness of demand-side policies in reducing unemployment.
-
Using real-world examples, evaluate the effectiveness of foreign aid in promoting economic growth and economic development.
-
Return to the facts of problem 30. Assume that Show Corporation is organized as an S corporation. In its second year of operations, Show has an operating loss of $40,000 and pays out $20,000 in...
-
The activities listed in lines 2125 serve primarily as examples of A) Underappreciated dangers B) Intolerable risks C) Medical priorities D) Policy failures
-
Draw the structure of cis-3,4-diethylcyclobutene. (a) Conrotatory ring opening produces only one product. Draw the product and determine whether its formation is best achieved under thermal...
-
Identify whether the product obtained from each of the following reactions is a meso compound or a pair of enantiomers: (a) Irradiation of (2E,4Z,6Z )-4,5-dimethyl-2,4,6-octatriene with UV light (b)...
-
For each of the following reactions, use brackets and two numbers to identify the type of sigmatropic rearrangement taking place: (a) (b) Heat TH. Heat
-
Prepare adjusting entries as of December 3 1 of the current year for the following transactions. Note: If no entry is required for a transaction / event , select " No journal entry required" in the...
-
If there were a shift of $8,000 in sales revenue from the banquet area to the dining room, would you expect the restaurant's overall operating income to increase or decrease? Explain your reasoning...
-
The balance sheets for Monitor World Corporation and additional information are provided below. MONITOR WORLD CORPORATION Balance Sheets December 3 1 , 2 0 2 4 and 2 0 2 3 2 0 2 4 2 0 2 3 Assets...

Study smarter with the SolutionInn App


