Draw the Lewis structure of boric acid, B(OH) 3 . (a) Is resonance important for its description?
Question:
Draw the Lewis structure of boric acid, B(OH)3.
(a) Is resonance important for its description?
(b) The proton transfer equilibrium for boric acid is given in a footnote to Table 6C.1. In that reaction does boric acid act as a Lewis acid, a Lewis base, or neither?
Justify your answer by using Lewis structures of boric acid and its conjugate base.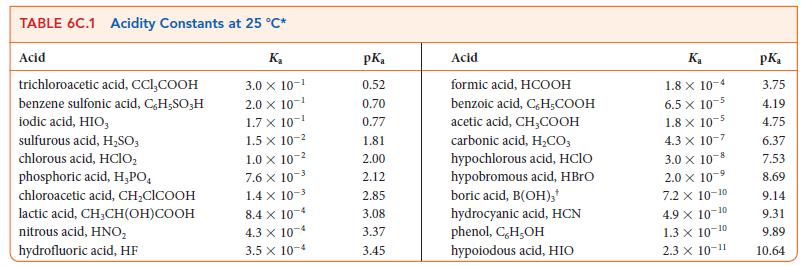
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 °C* Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C,H-SO;H iodic acid, HIO, sulfurous acid, H₂SO3 chlorous acid, HClO₂ phosphoric acid, H,PO chloroacetic acid, CH₂ClCOOH lactic acid, CH,CH(OH)COOH nitrous acid, HNO₂ hydrofluoric acid, HF K₂ 3.0 X 10-¹ 2.0 × 10-¹ 1.7 X 10-¹ 1.5 X 10-² 1.0 x 10-² 7.6 X 10-³ 1.4 x 10-³ 8.4 x 10-4 4.3 x 10-4 3.5 x 10-4 pK₂ 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C, H,COOH acetic acid, CH₂COOH carbonic acid, H₂CO3 hypochlorous acid, HClO hypobromous acid, HBrO boric acid, B(OH)3* hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO Ka 1.8 x 10-4 6.5 x 10-5 1.8 x 10-5 4.3 X 10-7 3.0 × 10-8 2.0 x 10-5 -9 7.2 x 10-10 4.9 X 10-¹ -10 1.3 × 10-10 2.3 × 10-11 pK₂ 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The Lewis structure of boric acid is H HOBOH HO Boric ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In the vapor phase, BeCl2 exists as a discrete molecule. (a) Draw the Lewis structure of this molecule, using only single bonds. Does this Lewis structure satisfy the octet rule? (b) What other...
-
The following items are related to income tax expenses. Determine the specific eight or nine-digit codification citation (XXX-XX-XX-XX) for each of the following items. Please enter only the ASC...
-
Hydrogen peroxide, H 2 O 2 , is a nontoxic bleaching agent being used as a replacement for chlorine in industry and home laundries. The bleaching process is an oxidation, and when hydrogen peroxide...
-
trade on the common stock of Taz, Inc. that have a strike price of $ 51.00 and a premium of $ 1.00 . In each of the next four parts, calculate the net profit (or loss) on the option position. Note:...
-
Strategy balanced scorecard. Meredith Corporation makes a special-purpose machine, D4H, used in the textile industry. Meredith has designed the D4H machine for 2009 to be distinct from its...
-
U.S. Metallurgical Inc. reported the following balances in its financial statements and disclosure notes at December 31, 2010. U.S.M.'s actuary determined that 2011 service cost is $60,000. Both the...
-
What are the advantages and disadvantages of judgmental sampling?
-
McTaggart-Hicks transactions as operating (O), investing (I), financing (F), non-cash investing and financing (NIF), or a transaction that is not reported on the statement of cash flows (N). Indicate...
-
I just need help with the last two rows, ram angular velocity and ram angular acceleration. I have the rest of the sheet filled out, what equations should I use for the last 2 rows ? Hint(s). ? To...
-
Draw the Lewis structure or symbol for each of the following species and identify each one as a Lewis acid or Lewis base: (a) NH3; (b) BF3; (c) Ag; (d) F; (e) H.
-
The pH of 0.40 m HF(aq) is 1.93. Calculate the change in pH when 0.356 g of sodium fluoride is added to 50.0 mL of the solution. Ignore any change in volume.
-
This problem is based on the appendix to this chapter. The Eastman Kodak Corporation had an issue of convertible bonds outstanding in spring 2005 that had a coupon rate of interest and sold for $...
-
What approach provides for the scalability of Scrum?
-
What type of problems are Scrum (Agile) projects best suited for?
-
Facebook is planning to change the layout of its homepage for user accounts and wants to test the new layout before releasing it. What type of experiment would be most suitable and how would it be...
-
What would you consider to be the right length of sprint for your project? Discuss with your group and present your discussion to the wider class.
-
What is measurement?
-
James and Quigley, LLC, is a CPA firm that offers both tax and audit services to its clients. The firm bills clients for cost plus 10%. It is straightforward to trace the direct costs associated with...
-
Find a least expensive route, in monthly lease charges, between the pairs of computer centers in Exercise 11 using the lease charges given in Figure 2. a) Boston and Los Angeles b) New York and San...
-
Propose a plausible mechanism for each of the following transformations: (a) (b) I-CI AICI3 CH,Cl, AICI,
-
Propose a plausible mechanism for the following transformation: NaOMe + Nacl OMe CI
-
Predict the product(s) of the following reactions: (a) (b) (c) (d) 1) HNO3, H,SO, 2) Zn, HCI Br
-
As a financial analyst of Bintang Bulan Berhad, you are required to analyse the company's financial performance. The financial statements of the company are as follows: BINTANG BULAN BERHAD STATEMENT...
-
Within the context of financial globalization and cross-border capital flows, what are the implications of interconnectedness and interdependence among global financial institutions for financial...
-
Draw the shear diagram for the beam. Set P = 600 lb, a = 5 ft, b = 7 ft. Draw the moment diagram for the beam.

Study smarter with the SolutionInn App


