Question: Given the following data: P4(s) + 6Cl(g) 4PCl3(g) P4(s) + 50(g) P4O10(S) PC13(g) + Cl(g) PCls (g) PC13(g) + O(g) ClPO(g) calculate AH for the
Given the following data: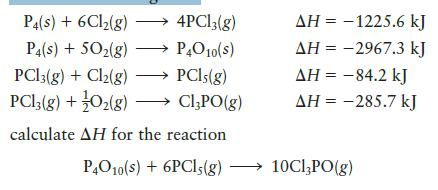
P4(s) + 6Cl(g) 4PCl3(g) P4(s) + 50(g) P4O10(S) PC13(g) + Cl(g) PCls (g) PC13(g) + O(g) ClPO(g) calculate AH for the reaction P4010(s) + 6PC15(g) AH = -1225.6 kJ AH = -2967.3 kJ AH = -84.2 kJ AH = -285.7 kJ 10ClPO(g)
Step by Step Solution
3.33 Rating (162 Votes )
There are 3 Steps involved in it
Given data Eq1 P4s 6Cl2g 4PCl3 H12256 KJ Eq2 P4s 5O2g P4O10s H29673 KJ ... View full answer

Get step-by-step solutions from verified subject matter experts


