Question: Given the following data, calculate the value for the overall formation constant for (mathrm{Mn}left(mathrm{C}_{2} mathrm{O}_{4}ight)_{2}{ }^{2-}) : [K=frac{left[mathrm{Mn}left(mathrm{C}_{2} mathrm{O}_{4}ight)_{2}^{2-}ight]}{left[mathrm{Mn}^{2+}ight]left[mathrm{C}_{2} mathrm{O}_{4}{ }^{2-}ight]^{2}}] Mn+ (aq) + C04
Given the following data,
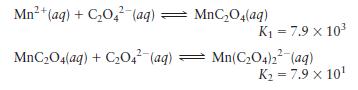
calculate the value for the overall formation constant for \(\mathrm{Mn}\left(\mathrm{C}_{2} \mathrm{O}_{4}ight)_{2}{ }^{2-}\) :
\[K=\frac{\left[\mathrm{Mn}\left(\mathrm{C}_{2} \mathrm{O}_{4}ight)_{2}^{2-}ight]}{\left[\mathrm{Mn}^{2+}ight]\left[\mathrm{C}_{2} \mathrm{O}_{4}{ }^{2-}ight]^{2}}\]
Mn+ (aq) + C04 (aq): MnCO4(aq) + C204 (aq) MnCO4(aq) K = 7.9 x 10 Mn(CO4)2 (aq) K = 7.9 x 10
Step by Step Solution
3.29 Rating (161 Votes )
There are 3 Steps involved in it
The formation constant for the reaction Mn C2O4 MnC2O4 is K 79 x 10 The formation con... View full answer

Get step-by-step solutions from verified subject matter experts


