K sp for Ni(OH) 2 is 6.5 * 10 18 . Use this value and data from
Question:
Ksp for Ni(OH)2 is 6.5 * 10–18. Use this value and data from Appendix 2B to calculate E° for the half-reaction Ni(OH)2(s) + 2 e– → Ni(s) + 2 OH–(aq).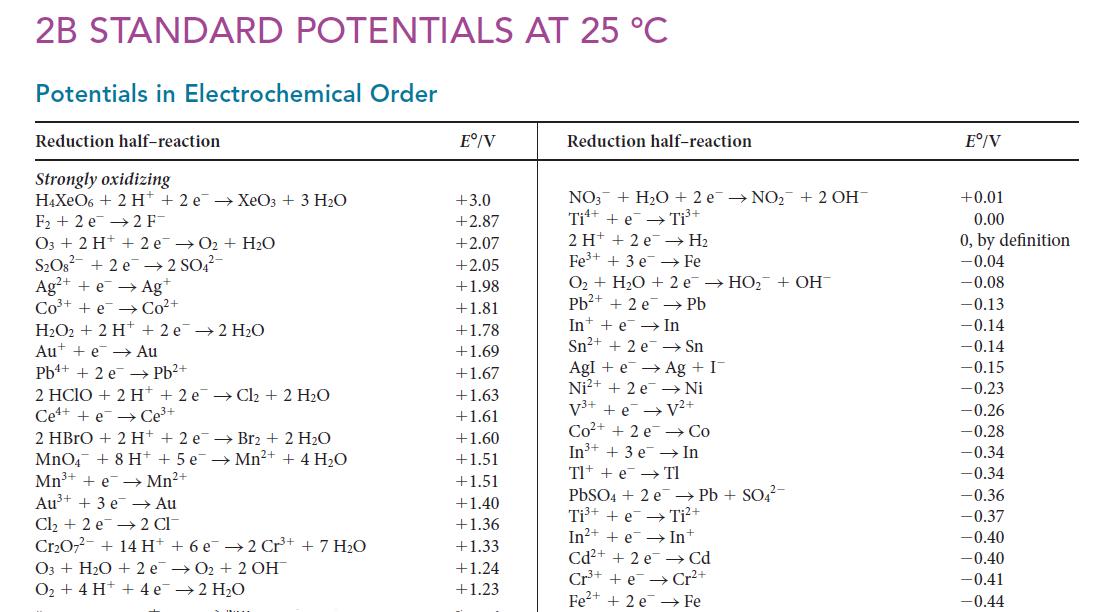
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 °C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+ 2 e XeO3 + 3 H₂O F₂2 e 2 F- O3 + 2H+ + 2 e→O₂ + H₂O S₂O8² +2e → 2 SO4²- Ag²+ + e → Ag+ CO³+ +e Co²+ H₂O2 + 2 H+ 2e → 2 H₂O Aue Au Pb²+ Pb+ + 2 e 2 HCIO + 2 H+ 2 eCl₂ + 2 H₂O Cee Ce³+ →Mn²+ + 4H₂O 2 HBrO + 2 H+ + 2 e Br2 + 2 H₂O MnO4 + 8 H+ + 5 e Mn³+ + e→ Mn²+ Au³+ + 3 e →→ Au Cl₂ +2 e 2 CI Cr₂O7² + 14 H+ + 6 e 2 Cr³+ + 7 H₂O O3+ H₂O + 2e O₂ + 4 H+ + 4e →O₂+ 2 OH → 2 H₂O Eº/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +1.23 Reduction half-reaction NO3 + H₂O + 2e →NO₂+ 2 OH Ti + e Ti³+ 2 H+ + 2e Fe³+ + 3 e → H₂ Fe O₂ + H₂O + 2e →HO₂ + OH Pb²+ + 2 e Pb In e In Sn²+ + 2 e Sn Agle → Ag + I Ni²+ + 2e → Ni V³+ + e → V²+ Co²+ +2e In³+ + 3 e Tl + e PbSO4 + 2e Ti³+ + e In²+ + e→→ Cd²+ + 2 e Cr³+e Fe²+ + 2 e → Co In Tl →→ Pb+ SO4²- Ti²+ In+ Cd Cr²+ Fe Eº/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41 -0.44
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Appendix 2B Write the solubility equilibrium NiOH2s ...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use only the data in Appendix 2B to calculate the acidity constant of HClO in water. 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing...
-
K sp for Cu(IO 3 ) 2 is 1.4 * 10 7 . Using this value and data in Appendix 2B, calculate E for the half-reaction Cu(IO 3 ) 2 (s) + 2 e Cu(s) + 2 IO 3 (aq). 2B STANDARD POTENTIALS AT 25 C...
-
(a) Use data from Appendix 2B to calculate the solubility product of Hg 2 Cl 2 . (b) Compare this number with the value listed in Table 6I.1 and comment on any difference. TABLE 61.1 Solubility...
-
Lead nitrate solution is added to a test tube containing potassium iodide solution. (a) Write the name and colour of the compound precipitated. (b) Write the balanced chemical equation for the...
-
Transfer pricing, goal congruence. The Orsilo Corporation makes end sells 10,000 multisystem music players each year Its Assembly Division purchases components from other divisions of Orsilo or from...
-
Swathmore Clothing Corporation grants its customers 30 days' credit. The company uses the allowance method for its uncollectible accounts receivable. During the year, a monthly bad debt accrual is...
-
What are the key dimensionless parameters? Integrate the equation to find an expression for \(\lambda / L\) as a function of dimensionless time. Is a steady state reached in this system?
-
Your friend, Diane Kiefner, teaches elementary school and operates her own wilderness kayaking tours in the summers. She thinks she has been doing fine financially, but has never really measured her...
-
what is the amount of the difference between the variable costing and absorption costing net operating income (losses) If the sales volumes in the east and west regions have been reversed what would...
-
A galvanic cell functions only when the electrical circuit is complete. In the external circuit the current is carried by the flow of electrons through a metal wire. Explain how the current is...
-
A galvanic cell has the following cell reaction: M(s) + 2 Zn 2+ (aq) 2 Zn(s) + M 4+ (aq). The standard potential of the cell is 10.16 V. What is the standard potential of the M 4+ /M redox couple?
-
A mixing chamber with heat transfer receives 2 kg/s of R-22 at 1 MPa, 40C in one line and 1 kg/s of R-22 at 30C, quality 50% in a line with a valve. The outgoing flow is at 1 MPa, 60C. Find the rate...
-
What factors should voters consider in deciding if a prosecutor should be reelected?
-
The construction schedule is the only project document that fully communicates the contractor's intentions for delivering the contracted scope of services over the full course of the project...
-
A construction contract differs from contracts that we generally deal with that focus on an easily defined physical object because the physical object can be examined. How is the object of a...
-
Many contracts require that the contractor prepare and present the construction schedule and receive the owner's approval before any contract payments are made. What are the three elements of the...
-
What is the basic objective of resource management?
-
Antiquities, Ltd., produces antique-looking books. Management has just received a request for a special order for 1,000 books and must decide whether to accept it. Venus Company, the purchaser, is...
-
Horse serum containing specific antibody to snake venom has been a successful approach to treating snakebite in humans. How do you think this anti-venom could be generated? What are some advantages...
-
Using your results from Problem P5.7, calculate q, ÎU, and ÎH for each step in the cycle and for the total cycle described in Figure 5.2. Figure 5.2 Isothermal expansion Pa Adiabatic cold...
-
At the transition temperature of 95.4C, the enthalpy of transition from rhombic to monoclinic sulfur is 0.38 kJ mol 1 . a. Calculate the entropy of transition under these conditions. b. At its...
-
One mole of a van der Waals gas at 25.0????C is expanded isothermally and reversibly from an initial volume of 0.010 m 3 to a final volume of 0.095 m 3 . For the van der Waals gas, (U/V)T = a/V 2 m ....
-
5. Solve the linear inequality. Graph the solution set on a number line. 7(x+4)-13 < 12 + 13(3 + x)
-
Can you delineate the role of central banks as key stakeholders in the financial ecosystem, elucidating their functions as monetary authorities responsible for price stability, lender of last resort...
-
write a presentation about a caf. -agenda -survey -presentation -staff, customer feedback add pictures

Study smarter with the SolutionInn App


