Question: (a) Use data from Appendix 2B to calculate the solubility product of Hg 2 Cl 2 . (b) Compare this number with the value listed
(a) Use data from Appendix 2B to calculate the solubility product of Hg2Cl2.
(b) Compare this number with the value listed in Table 6I.1 and comment on any difference.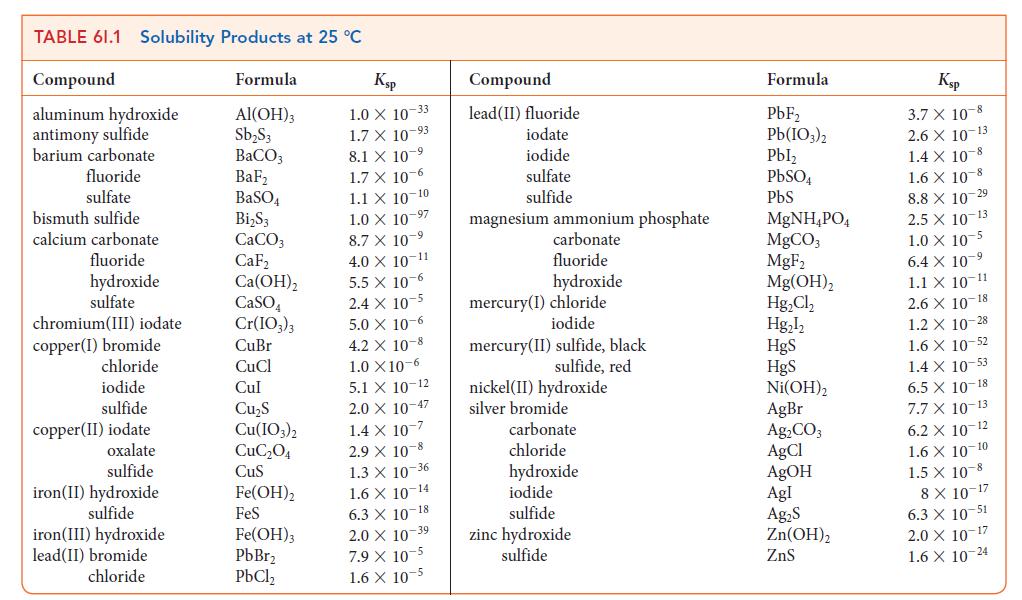
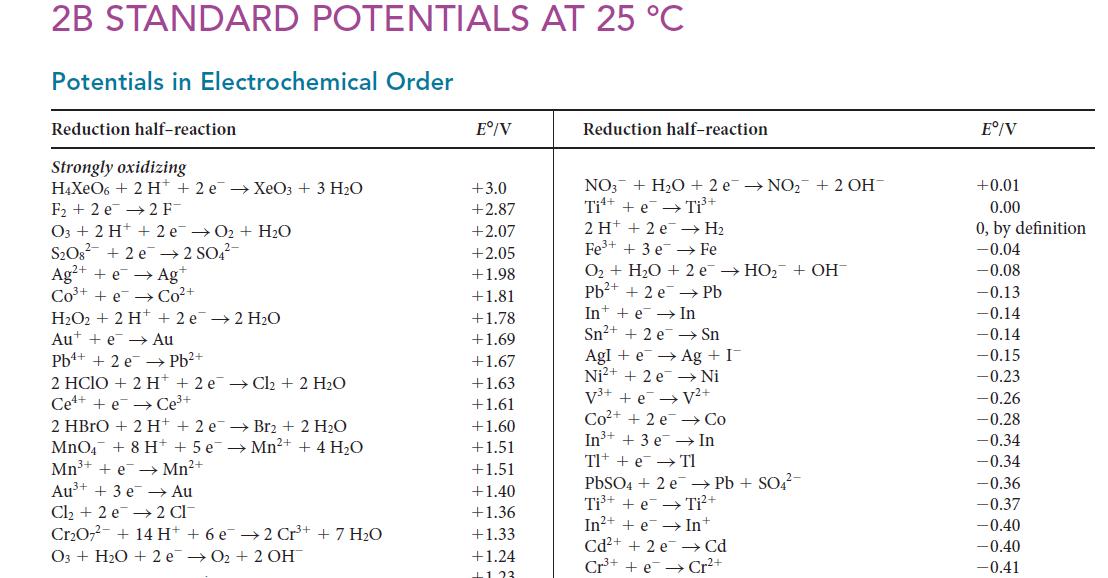
TABLE 61.1 Solubility Products at 25 C Compound aluminum hydroxide antimony sulfide barium carbonate fluoride sulfate bismuth sulfide calcium carbonate fluoride hydroxide sulfate chromium(III) iodate copper (1) bromide chloride iodide sulfide copper(II) iodate oxalate sulfide iron(II) hydroxide sulfide iron(III) hydroxide lead(II) bromide chloride Formula Al(OH)3 SbS3 BaCO3 BaF BaSO4 BiS3 CaCO3 CaF Ca(OH) CaSO4 Cr(IO3)3 CuBr CuCl Cul CuS Cu(IO3)2 CuC04 CuS Fe(OH)2 FeS Fe(OH)3 PbBr PbCl Ksp 1.0 X 10-33 1.7 X 10-93 8.1 X 107 1.7 x 10-6 1.1 X 10-10 1.0 X 10-97 8.7 X 10-9 4.0 X 10-11 5.5 x 10-6 2.4 x 10-5 5.0 x 10-6 4.2 X 10-8 1.0 X10-6 5.1 X 10-12 2.0 X 10-47 1.4 x 10-7 2.9 X 10-8 1.3 X 10-36 1.6 X 10-14 6.3 X 10-18 2.0 X 10 39 7.9 X 10-5 1.6 X 10-5 Compound lead(II) fluoride iodate iodide sulfate sulfide magnesium ammonium phosphate carbonate fluoride hydroxide mercury(I) chloride iodide mercury (II) sulfide, black sulfide, red nickel (II) hydroxide silver bromide carbonate chloride hydroxide iodide sulfide zinc hydroxide sulfide Formula PbF Pb(103)2 Pbl PbSO4 PbS MgNHPO4 MgCO3 MgF Mg(OH) HgCl Hgl HgS HgS Ni(OH)2 AgBr AgCO3 AgCl AgOH Agl AgS Zn(OH) ZnS Ksp 3.7 X 10-8 2.6 X 10-13 1.4 X 10-8 1.6 X 10 8 8.8 X 10-29 2.5 X 10-13 1.0 X 10-5 6.4 X 10 1.1 X 10-11 2.6 X 10-18 1.2 X 10-28 1.6 X 10-52 1.4 x 10-53 6.5 X 10-18 7.7 X 10-13 6.2 X 10-12 1.6 X 10-10 1.5 X 10-8 8 X 10-17 6.3 X 10-51 2.0 X 10-17 1.6 X 10-24
Step by Step Solution
3.24 Rating (162 Votes )
There are 3 Steps involved in it
a K sp 12 10 17 b The cal... View full answer

Get step-by-step solutions from verified subject matter experts


