Question:
Methanol, CH3OH, is a clean-burning liquid fuel being used as a replacement for gasoline. Calculate the theoretical yield in kilograms of CO2 produced by the combustion of 1.00 L of methanol (of density 0.791 g · cm–3) and compare it with the 2.16 kg of CO2 generated by the combustion of 1.00 L of octane. Which fuel contributes more CO2 per liter to the atmosphere when burned?
What other factors would you take into consideration when deciding which of the two fuels to use? See Box 8B.1.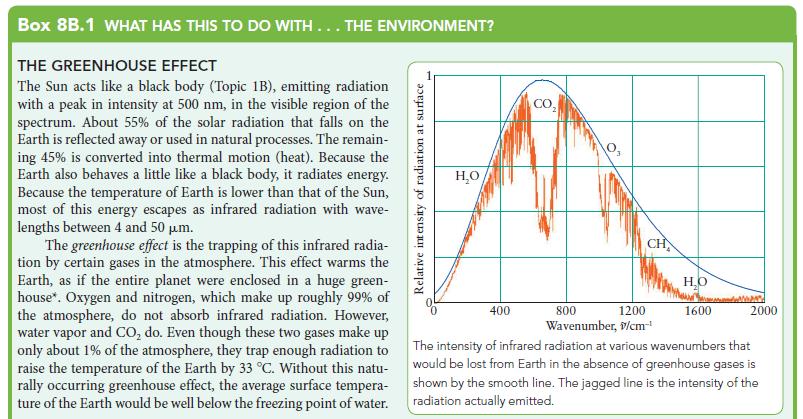
Transcribed Image Text:
Box 8B.1 WHAT HAS THIS TO DO WITH ...THE ENVIRONMENT?
THE GREENHOUSE EFFECT
The Sun acts like a black body (Topic 1B), emitting radiation
with a peak in intensity at 500 nm, in the visible region of the
spectrum. About 55% of the solar radiation that falls on the
Earth is reflected away or used in natural processes. The remain-
ing 45% is converted into thermal motion (heat). Because the
Earth also behaves a little like a black body, it radiates energy.
Because the temperature of Earth is lower than that of the Sun,
most of this energy escapes as infrared radiation with wave-
lengths between 4 and 50 μm.
The greenhouse effect is the trapping of this infrared radia-
tion by certain gases in the atmosphere. This effect warms the
Earth, as if the entire planet were enclosed in a huge green-
house*. Oxygen and nitrogen, which make up roughly 99% of
the atmosphere, do not absorb infrared radiation. However,
water vapor and CO₂ do. Even though these two gases make up
only about 1% of the atmosphere, they trap enough radiation to
raise the temperature of the Earth by 33 °C. Without this natu-
rally occurring greenhouse effect, the average surface tempera-
ture of the Earth would be well below the freezing point of water.
Relative intensity of radiation at surface
H₂O
400
CO₂
8
X
CH
800
1200
Wavenumber, >/cm-¹
H₂O
1600
www.
2000
The intensity of infrared radiation at various wavenumbers that
would be lost from Earth in the absence of greenhouse gases is
shown by the smooth line. The jagged line is the intensity of the
radiation actually emitted.










