Reactions between gases in the atmosphere are not at equilibrium, but for a thorough understanding of them
Question:
Reactions between gases in the atmosphere are not at equilibrium, but for a thorough understanding of them we need to study both the rates at which they take place and their behavior under equilibrium conditions.
(a) The equilibrium for the depletion of ozone in the stratosphere is summarized by the equation 2 O3(g) ⇌ 3 O2(g). From values in Appendix 2A, determine the standard Gibbs free energy and the standard entropy for the reaction.
(b) What is the equilibrium constant of the reaction in part (a) at 25°C? What is the significance of your answer for ozone depletion?
(c) A reaction that destroys ozone in the stratosphere is O3(g) + O(g) ⇌ 2 O2(g). Calculate the value of the equilibrium constant for this reaction at 25°C, given that at that temperature the reaction is catalyzed (accelerated) by NO2 molecules in a two-step process: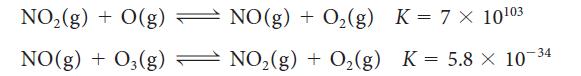
(d) Use your answer to part (c) to find the standard Gibbs free energy of formation of O atoms.
(e) The temperature dependence of the equilibrium constant of the reaction N2(g) + O2(g) ⇌ 2 NO(g), which makes an important contribution to the concentration of atmospheric nitrogen oxides, can be expressed as ln K = 2.5 – (21 700 K)/T. What is the standard enthalpy of the forward reaction at 298 K? Will this reaction proceed further at the very low temperatures of the stratosphere or at the very high temperatures in an internal combustion engine?
(f) An equimolar mixture of N2 and O2 was heated to a certain temperature until the reaction in part (e) came to equilibrium. The equilibrium reaction mixture was found to contain an equal number of moles of each reactant and product. At what temperature was the reaction carried out?
(g) An equimolar mixture of N2 and O2 with a total pressure of 4.00 bar was allowed to come to equilibrium in the reaction in part (e) at 1200. K. What will be the partial pressure of each reactant and product at equilibrium?
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





