Sports drinks provide water to the body in the form of an isotonic solution (one having the
Question:
Sports drinks provide water to the body in the form of an isotonic solution (one having the same osmotic pressure as human blood). These drinks contain electrolytes such as NaCl and KCl as well as sugar and flavoring. One of the main flavoring agents in sport drinks is citric acid (1).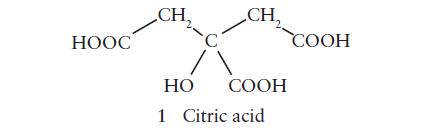
(a) Indicate the hybridization of each C atom in citric acid.
(b) Can citric acid take part in hydrogen bonding?
(c) Predict from a consideration of intermolecular forces whether citric acid is a gas, liquid, or solid at 25°C and whether it is soluble in water.
(d) Normal saline solution is an isotonic solution (Topic 5F) containing 0.9% NaCl by mass in water. Assuming complete dissociation of the NaCl, what is the total molar concentration of all solutes in an isotonic solution? Assume a density of 1.00 g · cm–3 for the solution.
(e) If you decide to make up 500.0 mL of a sports drink with 1.0 g of NaCl and glucose, what mass of glucose do you need to add to the NaCl and water to make the solution isotonic (see part d)? Assume a density of 1.00 g · cm–3 for the solution.
(f) A paramedic treating injuries in a remote area has 300.0 mL of a 1.00% by mass solution of boric acid, B(OH)3, that needs to be made isotonic (assume that the density is 1.00 g · cm–3). What mass of NaCl should be added? Assume that the NaCl is completely dissociated in the solution and that the volume of the solution does not change upon addition of the NaCl. Take into account the 0.007% deprotonation of boric acid.
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





