State the molar solubility in water of (a) O 2 at 50. kPa; (b) CO 2 at
Question:
State the molar solubility in water of
(a) O2 at 50. kPa;
(b) CO2 at 500. Torr;
(c) CO2 at 0.10 atm. The temperature in each case is 20°C, and the pressures are partial pressures of the gases.
Use the information in Table 5D.2.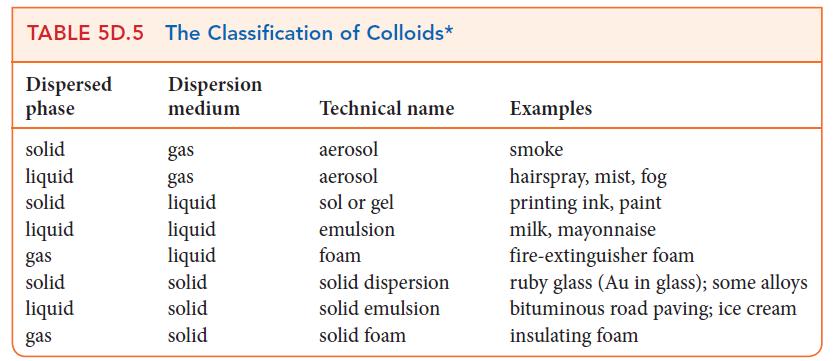
Transcribed Image Text:
TABLE 5D.5 The Classification of Colloids* Dispersed phase solid liquid solid liquid gas solid liquid gas Dispersion medium gas gas liquid liquid liquid solid solid solid Technical name aerosol aerosol sol or gel emulsion foam solid dispersion solid emulsion solid foam Examples smoke hairspray, mist, fog printing ink, paint milk, mayonnaise fire-extinguisher foam ruby glass (Au in glass); some alloys bituminous road paving; ice cream insulating foam
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a 64 10 ...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Identify and classify any additional risk factors that came to light through the information in Part II of the case Do not repeat factors already identified in Part I Document the new risk factors...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
True or False The slope of the line 2y = 3x + 5 is 3.
-
The need to remove organic pollutants from wastewater is common to many industrial processes. Separation methods that may be considered are: (1) adsorption, (2) distillation, (3) liquid-liquid...
-
Mark Hurd has just assumed the top job at H-P. He has asked you as a staff VP to draw up a course of action to get the ailing PC division up to competitive parity with Dell. If you need to make some...
-
The calculation of solar radiation impinging on a surface is of importance in many applications, for example, design of solar collectors, temperature control of buildings, etc. Your goal is to review...
-
New Business Ventures, Inc., has an outstanding perpetual bond with a 9 percent coupon rate that can be called in one year. The bond makes annual coupon payments and has a par value of $1,000. The...
-
Pancake Village had sales of $1.5 million with depreciation of $350,000 and other operating costs that ran 35% of sales. They paid $180,000 in dividends with a tax rate of 40% and interest expense of...
-
A bank decides to create a five-year principal-protected note on a non-dividend-paying stock by offering investors a zero-coupon bond plus a bull spread created from calls. The risk-free rate is 4%...
-
Most reactions proceed faster at higher temperatures, and many industrial processes are carried out at high temperatures. However, for exothermic reactions increasing the temperature reduces the...
-
In a gas-phase equilibrium mixture of PCl 5 , PCl 3 , and Cl 2 at 500. K, PPCl 5 = 1.18 bar, PCl 2 = 5.43 bar. What is the partial pressure of PCl 3 , given that K = 25 for the reaction PCl 5 (g) PCl...
-
Multinational firms domiciled in the United States do not have to pay U.S. income taxes on foreign earnings, unless those earnings are returned to the United States. Accordingly, the U.S. income tax...
-
Sardars recommendation for the next step should be to: A. review results from backtesting the strategy. B. make recommendations for rebalancing the portfolio. C. forecast companies performances and...
-
The most appropriate risk attribution approach for the fixed-income manager is to: A. decompose historical returns into a top-down factor framework. B. evaluate the marginal contribution to total...
-
In managing the fund at his previous employer, Deweys investment process can be best described as: A. an activist strategy. B. a top-down strategy. C. a bottom-up strategy. Jack Dewey is managing...
-
The Barboa Fund can be best described as a fund segmented by: A. size/style. B. geography. C. economic activity. Three years ago, the Albright Investment Management Company (Albright) added four new...
-
Based on Exhibit 1, the target semideviation for the portfolio is closest to: A. 2.78%. B. 3.68%. C. 4.35%. Alexandra Jones, a senior adviser at Federalist Investors (FI), meets with Erin Bragg, a...
-
Your company spent $5,000 last year on business related meals and entertainment. Calculate the difference between the cash flow and the deductibility of these expenses for tax purposes.
-
Fred Farmer needs to prepare a balance sheet for his bank. He spent the day getting the following information. Fred needs your help to build a balance sheet and evaluate it. The information was...
-
Identify the reagents you would use to accomplish each of the following transformation. a. b. c. d. e. f. H.
-
Predict the products for each of the following: a. b. c. d. e. f. 1) O3 2) DMS 3) Excess LAH 4) H20 1) O3 2) DMS 3) Excess LAH 4) H20
-
Explain why the oscillations in the two-phase coexistence region using the RedlichKwong and van der Waals equations of state (see Figure 7.4) do not correspond to reality. Figure 7.4 140 120 100 304...
-
ii) (2 points) For smooth f with a Lipshitz constant L, let F(y) = f(t + h, y + hf (t, y)), verify that F is also Lipshitz with some Liphsitz constant independent of h for h small (say less than...
-
This problem asks you to review the lecture notes with the proof of the Laurent series theorem and address certain key steps, but in the context of an example function 1 f(z) = x+1 Note, this f(z) is...
-
4. In the table at the left, N(n) is the number, in thousands, of Canadian home computers sold, where n is the number of years since 1990. a) Determine the equation that best models this...

Study smarter with the SolutionInn App


